This is a preprint.
Graft-versus-host disease prophylaxis shapes T cell biology and immune reconstitution after hematopoietic cell transplant
- PMID: 40061351
- PMCID: PMC11888538
- DOI: 10.1101/2025.02.25.25322901
Graft-versus-host disease prophylaxis shapes T cell biology and immune reconstitution after hematopoietic cell transplant
Abstract
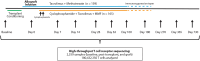
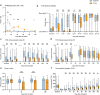
Successful hematopoietic cell transplant requires immunosuppression to prevent graft-versus-host disease (GVHD), a lethal, T-cell-mediated post-transplant complication. The phase 3 BMT CTN 1703 trial demonstrated superior GVHD-free/relapse-free survival for post-transplant cyclophosphamide (PT-Cy)-based GVHD prophylaxis versus tacrolimus/methotrexate (Tac/MTX), but did not improve overall survival. To compare T-cell biology between GVHD prophylaxis regimens, 324 patients were co-enrolled onto BMT CTN 1801 (NCT03959241). We quantified T-cell immune reconstitution using multi-modal analysis, including T-cell receptor (TCR) sequencing of 2,359 longitudinal samples (180,432,350 T-cells). Compared to Tac/MTX, PT-Cy was associated with an early, substantial reduction in TCR diversity that was sustained for 2 years. PT-Cy led to a T-cell reconstitution bottleneck, including reduced thymic output and virus-associated TCRs. Decreased D+14 TCR diversity predicted prevention of chronic GVHD, but also correlated with increased moderate-to-severe infections. This study reveals how distinct immunosuppression strategies have significant effects on the global immune repertoire, underpinning post-transplant clinical outcomes.
Figures



References
-
- Powles R.L., et al. Cyclosporin A to prevent graft-versus-host disease in man after allogeneic bone-marrow transplantation. Lancet 1, 327–329 (1980). - PubMed
-
- Storb R., et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N Engl J Med 314, 729–735 (1986). - PubMed
-
- Zeiser R. & Blazar B.R. Pathophysiology of Chronic Graft-versus-Host Disease and Therapeutic Targets. N Engl J Med 377, 2565–2579 (2017). - PubMed
-
- Shlomchik W.D. Graft-versus-host disease. Nat Rev Immunol 7, 340–352 (2007). - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
