Novel Mechanical Aspiration Thrombectomy in Patients With Acute Pulmonary Embolism: Results From the Prospective APEX-AV Trial
- PMID: 40061412
- PMCID: PMC11887559
- DOI: 10.1016/j.jscai.2024.102463
Novel Mechanical Aspiration Thrombectomy in Patients With Acute Pulmonary Embolism: Results From the Prospective APEX-AV Trial
Abstract
Background: There is a need for additional data to assess procedural efficacy and risks associated with mechanical thrombectomy for treating pulmonary embolism (PE) due to its increased utilization and diversity of patient populations presenting with PE. This study evaluated the safety and efficacy of percutaneous mechanical aspiration thrombectomy with the AlphaVac F1885 System (AngioDynamics) in patients with acute intermediate-risk PE.
Methods: Patients with acute intermediate-risk PE and a right ventricular (RV)/left ventricular (LV) diameter ratio of ≥0.9 were eligible for enrollment in this prospective, multicenter, single-arm study. The primary effectiveness end point was reduction in the RV/LV ratio at 48 hours. The primary safety end point was the rate of major adverse events (MAEs) defined as subjects who experienced major bleeding, device-related deaths, clinical deterioration, or pulmonary vascular or cardiac injury within 48 hours postprocedurally.
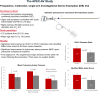
Results: In total, 122 subjects were enrolled at 25 sites. Mean procedure time was 37.2 ± 17.7 minutes. There were statistically significant reductions in mean 48-hour postprocedural RV/LV diameter ratio (-0.45 ± 0.27; P < .001). Postprocedural mean pulmonary arterial pressure also significantly declined from 27.8 ± 7.8 mm Hg before the procedure to 21.8 ± 7.2 mm Hg (P < .001). There was a 35.5% mean reduction in clot burden as measured by the modified Miller index score. Five (4.1%) subjects developed 7 MAEs during the postprocedural 48-hour assessment period, the majority of which were access site bleeding.
Conclusions: Percutaneous mechanical aspiration thrombectomy with the AlphaVac system provided a safe and effective treatment for acute intermediate-risk PE with a significant reduction in RV/LV ratio and clot burden with a low rate of adverse events.
Keywords: AlphaVac; aspiration thrombectomy; intermediate-risk pulmonary embolism; mechanical thrombectomy; thrombus; vacuum aspiration.
© 2024 The Author(s).
Conflict of interest statement
Mona Ranade has received consulting fees from AngioDynamics, Medtronic, Boston Scientific, Asahi Intecc. Malcolm T. Foster, Sabah Butty, Taral Patel, and John Moriarty have received consulting fees from AngioDynamics. David Zlotnick has received consulting fees from Inari Medical and AngioDynamics. Dean Ferrera has received consulting fees from AngioDynamics, Surmodics, Philips, Medtronic, CSI, and Shockwave Medical. Brian Stegman has received consulting fees from Boston Scientific, Inari Medical, Inquis Medical, Edwards Life Sciences, and Medtronic. Sukhdeep Basra has received consulting fees from AngioDynamics, Lexicon, and Abbott. Brent Keeling has received consulting fees from AngioDynamics and Penumbra.
Figures



References
-
- Giri J., Sista A.K., Weinberg I., et al. Interventional therapies for acute pulmonary embolism: current status and principles for the development of novel evidence: a scientific statement from the American Heart Association. Circulation. 2019;140(20):e774–e801. doi: 10.1161/CIR.0000000000000707. - DOI - PubMed
-
- Moriarty J.M., Rueda V., Liao M., et al. Endovascular removal of thrombus and right heart masses using the AngioVac System: Results of 234 patients from the prospective, multicenter Registry of AngioVac Procedures in Detail (RAPID) J Vasc Interv Radiol. 2021;32(4):549–557.e3. doi: 10.1016/j.jvir.2020.09.012. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

