Specific Genetic Mutations Impact Chemotherapy Resistance and Therapeutic Efficacy of Oncolytic Viruses in Ovarian Cancer
- PMID: 40062380
- PMCID: PMC12485382
- DOI: 10.1158/1535-7163.MCT-24-0906
Specific Genetic Mutations Impact Chemotherapy Resistance and Therapeutic Efficacy of Oncolytic Viruses in Ovarian Cancer
Abstract
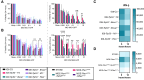
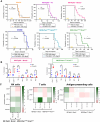
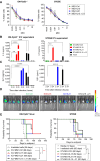
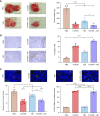
Epithelial ovarian cancer (EOC) is the most lethal gynecologic cancer, and those affected are in urgent need of new therapeutic strategies. Standard treatment is surgery followed by taxane- and platinum-based chemotherapy. However, the rate of relapse is high, and the 5-year survival is only 45%. Oncolytic viruses (OV) are a promising approach to EOC therapy through remodeling the immune composition of the tumor microenvironment. Treatment response in EOC tumors can differ based on the presence of key tumorigenic mutations. This study evaluated the impact of specific tumor mutations on the response to the current standard-of-care carboplatin, two promising OV candidates VSVΔM51 and MG1, an infected cell vaccine (ICV-MG1) regimen, and the antiangiogenic drug Fc3TSR. Mice with tumors harboring constitutive K-Ras activation showed an enhanced response to carboplatin and VSVΔM51 treatment. Additionally, VSVΔM51 treatment prolonged survival of syngeneic mice bearing tumors with mutations in Pten and Kras, Pten and Trp53, or Trp53 and Brca2 with increased activation of CD4+ and CD8+ T lymphocytes in the peritoneal tumor microenvironment. To enhance OV potency, an MG1-based infected cell vaccine inducing the expression of IL21 or IL15 + IL21 was developed and found to enable strong and long-lasting antitumoral immunity in two carboplatin-refractory syngeneic models, ID8-Trp53-/- and STOSE. VSVΔM51 combined with the antiangiogenic Fc3TSR enhanced efficacy in the ID8 model. In summary, OV-based immunotherapy has shown promise in diverse murine models of EOC-bearing clinically relevant mutations, thus laying the foundation for developing new OV-based strategies to target a large spectrum of EOC genotypes.
©2025 The Authors; Published by the American Association for Cancer Research.
Conflict of interest statement
A.O. Cudmore reports grants from Ovarian Cancer Canada and Canadian Institutes of Health Research during the conduct of the study. H. Murshed reports grants from Ottawa Hospital Research Institute during the conduct of the study. E.A. Macdonald reports grants from Canadian Institutes of Health Research and Ovarian Cancer Canada during the conduct of the study. M. Grondin reports grants from Canadian Institutes of Health Research and Ovarian Cancer Canada during the conduct of the study. B.C. Vanderhyden reports grants from Canadian Institutes of Health Research and Ovarian Cancer Canada during the conduct of the study. No disclosures were reported by the other authors.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

