Cinnabarinic acid protects against metabolic dysfunction-associated steatohepatitis by activating aryl hydrocarbon receptor-dependent AMPK signaling
- PMID: 40062565
- PMCID: PMC12258133
- DOI: 10.1152/ajpgi.00337.2024
Cinnabarinic acid protects against metabolic dysfunction-associated steatohepatitis by activating aryl hydrocarbon receptor-dependent AMPK signaling
Abstract
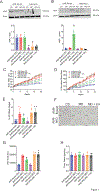
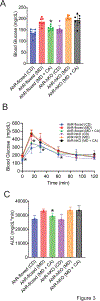
Metabolic dysfunction-associated steatohepatitis (MASH) is an advanced form of metabolic dysfunction-associated steatotic liver disease (MASLD) characterized by the accumulation of fats in the liver, chronic inflammation, hepatocytic ballooning, and fibrosis. This study investigates the significance of hepatic aryl hydrocarbon receptor (AhR) signaling in cinnabarinic acid (CA)-mediated protection against MASH. Here, we report that livers of high-fat, high-fructose, high-cholesterol diet-fed hepatocyte-specific aryl hydrocarbon receptor knockout mice (AhR-hKO) exhibited aggravated steatosis, inflammation, and fibrosis compared with control AhR-floxed livers. Moreover, treatment with a tryptophan catabolite, CA, reduced body weight gain and significantly attenuated hepatic steatosis, inflammation, ballooning, fibrosis, and liver injury only in AhR-floxed but not in AhR-hKO mice, strongly indicating that the CA-mediated protection against steatohepatitis is AhR-dependent. Furthermore, protection against lipotoxicity by CA-activated AhR signaling was confirmed by utilizing an in vitro human hepatocyte model of MASLD. Mechanistically, CA-induced AhR-dependent signaling augmented AMP-activated protein kinase (AMPK), leading to the upregulation of peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC1α) and attenuation of sterol regulatory element-binding protein-1 (SREBP1) to regulate hepatic lipid metabolism. Collectively, our findings indicate that CA-mediated protection against MASH is dependent on hepatic AhR signaling, and selective endogenous AhR agonists that regulate lipogenesis can serve as promising future therapeutics against MASLD.NEW & NOTEWORTHY The study showed that the absence of AhR in hepatocytes results in exacerbated metabolic dysfunction-associated steatohepatitis (MASH) in mice subjected to a Western-style high-fat, high-fructose, high-cholesterol diet. Moreover, treatment with a tryptophan catabolite, cinnabarinic acid (CA), mitigated hallmarks of MASH in an AhR-dependent manner. In conclusion, the study delineates the significance of hepatic AhR-dependent AMPK signaling in CA-mediated protection against MASH.
Keywords: aryl hydrocarbon receptor; cinnabarinic acid; liver; metabolic dysfunction-associated steatohepatitis; metabolic dysfunction-associated steatotic liver disease.
Conflict of interest statement
DISCLOSURES
The authors declare no conflict of interest.
Figures
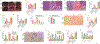

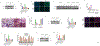

References
-
- Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, Anstee QM, Abdelmalek MF, Younossi Z, Baum SJ, Francque S, Charlton MR, Newsome PN, Lanthier N, Schiefke I, Mangia A, Pericas JM, Patil R, Sanyal AJ, Noureddin M, Bansal MB, Alkhouri N, Castera L, Rudraraju M, Ratziu V, and Investigators M-N. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. The New England journal of medicine 390: 497–509, 2024. - PubMed
-
- Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, Wang C, Patel G, Franks DG, Schlezinger J, Sherr DH, Silverstone AE, Hahn ME, and McCune JM. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PloS one 9: e87877, 2014. - PMC - PubMed
MeSH terms
Substances
Grants and funding
- R01 DK121951/DK/NIDDK NIH HHS/United States
- EY030513/HHS | NIH | National Eye Institute (NEI)
- DK122028/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
- R01 EY030513/EY/NEI NIH HHS/United States
- DK121951/HHS | NIH | National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

