Artificial Intelligence Versus Rules-Based Approach for Segmenting NonPerfusion Area in a DRCR Retina Network Optical Coherence Tomography Angiography Dataset
- PMID: 40062815
- PMCID: PMC11905605
- DOI: 10.1167/iovs.66.3.22
Artificial Intelligence Versus Rules-Based Approach for Segmenting NonPerfusion Area in a DRCR Retina Network Optical Coherence Tomography Angiography Dataset
Abstract
Purpose: Loss of retinal perfusion is associated with both onset and worsening of diabetic retinopathy (DR). Optical coherence tomography angiography is a noninvasive method for measuring the nonperfusion area (NPA) and has promise as a scalable screening tool. This study compares two optical coherence tomography angiography algorithms for quantifying NPA.
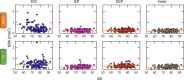
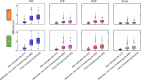
Methods: Adults with (N = 101) and without (N = 274) DR were recruited from 20 U.S. sites. We collected 3 × 3-mm macular scans using an Optovue RTVue-XR. Rules-based (RB) and deep-learning-based artificial intelligence (AI) algorithms were used to segment the NPA into four anatomical slabs. For comparison, a subset of scans (n = 50) NPA was graded manually.
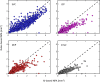
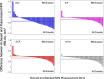
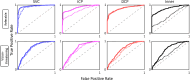
Results: The AI method outperformed the RB method in intersection over union, recall, and F1 score, but the RB method has better precision relative to manual grading in all anatomical slabs (all P ≤ 0.001). The AI method had a stronger rank correlation with Early Treatment of Diabetic Retinopathy Study DR severity than the RB method in all slabs (all P < 0.001). NPAs graded using the AI method had a greater area under the receiver operating characteristic curve for diagnosing referable DR than the RB method in the superficial vascular complex, intermediate capillary plexus, and combined inner retina (all P ≤ 0.001), but not in the deep capillary plexus (P = 0.92).
Conclusions: Our results indicate that output from the AI-based method agrees better with manual grading and can better distinguish between clinically relevant DR severity levels than a RB approach using most plexuses.
Conflict of interest statement
Disclosure:
Figures


References
-
- Wong TY, Sun J, Kawasaki R, et al. .. Guidelines on diabetic eye care: the International Council of Ophthalmology recommendations for screening, follow-up, referral, and treatment based on resource settings. Ophthalmology. 2018; 125(10): 1608–1622. - PubMed
-
- Antonetti DA, Klein R, Gardner TW.. Diabetic retinopathy. N Engl J Med. 2012; 366(13): 1227–1239. - PubMed
-
- Early Treatment of Diabetic Retinopathy Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—an extension of the modified airlie house classification: ETDRS report number 10. Ophthalmology. 1991; 98(5): 786–806. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

