High-Dose Vitamin D in Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MS Randomized Clinical Trial
- PMID: 40063041
- PMCID: PMC11894546
- DOI: 10.1001/jama.2025.1604
High-Dose Vitamin D in Clinically Isolated Syndrome Typical of Multiple Sclerosis: The D-Lay MS Randomized Clinical Trial
Abstract
Importance: Vitamin D deficiency is a risk factor for multiple sclerosis (MS) and is associated with the risk of disease activity, but data on the benefits of supplementation are conflicting.
Objective: To evaluate the efficacy of high-dose cholecalciferol as monotherapy in reducing disease activity in patients with clinically isolated syndrome (CIS) typical for MS.
Design, setting, and participants: The D-Lay MS trial was a parallel, double-blind, randomized placebo-controlled clinical trial in 36 MS centers in France. Patients were enrolled from July 2013 to December 2020 (final follow-up on January 18, 2023). Untreated patients with CIS aged 18 to 55 years with CIS duration less than 90 days, serum vitamin D concentration less than 100 nmol/L, and diagnostic magnetic resonance imaging (MRI) meeting 2010 criteria for dissemination in space or 2 or more lesions and presence of oligoclonal bands were recruited.
Intervention: Patients were randomized 1:1 to receive oral cholecalciferol 100 000 IU (n = 163) or placebo (n = 153) every 2 weeks for 24 months.
Main outcomes and measures: The primary outcome measure was disease activity, defined as occurrence of a relapse and/or MRI activity (new and/or contrast-enhancing lesions) over 24 months of follow-up, also analyzed as separate secondary outcomes.
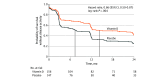
Results: Of the 316 participants enrolled and randomized (median [IQR] age, 34 [28-42] years; 70% women), the primary analysis included 303 patients (95.9%) who took at least 1 dose of the study drug and 288 (91.1%) ultimately completed the 24-month trial. Disease activity was observed in 94 patients (60.3%) in the vitamin D group and 109 patients (74.1%) in the placebo group (hazard ratio [HR], 0.66 [95% CI, 0.50-0.87]; P = .004), and median time to disease activity was longer in the vitamin D group (432 vs 224 days; log-rank P = .003). All 3 secondary MRI outcomes reported significant differences favoring the vitamin D group vs the placebo group: MRI activity (89 patients [57.1%] vs 96 patients [65.3%]; HR, 0.71 [95% CI, 0.53-0.95]; P = .02), new lesions (72 patients [46.2%] vs 87 patients [59.2%]; HR, 0.61 [95% CI, 0.44-0.84]; P = .003), and contrast-enhancing lesions (29 patients [18.6%] vs 50 patients [34.0%]; HR, 0.47 [95% CI, 0.30-0.75]; P = .001). All 10 secondary clinical outcomes showed no significant difference, including relapse, which occurred in 28 patients (17.9%) in the vitamin D group vs 32 (21.8%) in the placebo group (HR, 0.69 [95% CI, 0.42-1.16]; P = .16). Results were similar in a subset of 247 patients meeting updated 2017 diagnostic criteria for relapsing-remitting MS at treatment initiation. Severe adverse events occurred in 17 patients in the vitamin D group and 13 in the placebo group, none of which were related to cholecalciferol.
Conclusions and relevance: Oral cholecalciferol 100 000 IU every 2 weeks significantly reduced disease activity in CIS and early relapsing-remitting MS. These results warrant further investigation, including the potential role of pulse high-dose vitamin D as add-on therapy.
Trial registration: ClinicalTrials.gov Identifier: NCT01817166.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

