Ablation of symptomatic uterine fibroids with the Mirabilis system for rapid noninvasive ultrasound-guided high-intensity focused ultrasound (HIFU): a prospective observational clinical study
- PMID: 40063166
- PMCID: PMC12106475
- DOI: 10.1007/s11547-025-01972-6
Ablation of symptomatic uterine fibroids with the Mirabilis system for rapid noninvasive ultrasound-guided high-intensity focused ultrasound (HIFU): a prospective observational clinical study
Abstract
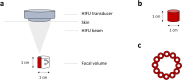
Objectives: Uterine fibroids often lead to symptoms that negatively impact health-related quality of life (HRQOL). High-intensity focused ultrasound (HIFU) has emerged as a promising noninvasive treatment for reducing fibroid size and symptoms. The Mirabilis system for ultrasound (US)-guided HIFU introduces a novel technique known as 'shell ablation'. This study evaluates the feasibility and efficacy of Mirabilis in a clinical setting, focusing on clinical outcomes.
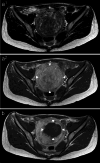
Materials and methods: Sixteen patients with 23 uterine fibroids were treated with the Mirabilis system. Follow-up assessments included US and MRI at baseline, 6 weeks, 3, 6 and 9 months, and 1 year after HIFU. Changes in symptoms and QOL were evaluated using the Uterine Fibroid Symptom and HRQOL Questionnaire.
Results: A significant reduction in fibroid volume was observed after HIFU (baseline 182.1 ± 49.3 ml; 1 year: 76.0 ± 37.9 ml, p < 0.001). The symptom severity score significantly declined (baseline 57.2 ± 3.8; 1 year: 30.2 ± 4.9, p < 0.001), correlating with a significant improvement in HRQOL (baseline 47.0 ± 3.9, 1 year: 71.8 ± 5.3, p < 0.001).
Conclusion: HIFU with the portable Mirabilis system is a feasible and safe noninvasive treatment for symptomatic uterine fibroids in an outpatient setting. This approach allows efficient and rapid ablation even for large fibroids, significantly reducing fibroid volume and symptoms.
Keywords: Fibroid-associated symptoms; High-intensity focused ultrasound; Mirabilis system; Mobile device; Shell ablation.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interests: The authors have no competing interests to declare that are relevant to the content of this article. Ethical approval: The study was conducted in accordance to the Declaration of Helsinki. It was approved by the local ethics committee of the University Hospital Bonn (No. 295/19), and it was registered in the German Clinical Trials Register (DRKS00020529). Written informed consent to participation was obtained from all patients.
Figures




References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

