Gilteritinib versus salvage chemotherapy in predominantly Asian patients with relapsed/refractory FLT3-mutated acute myeloid leukemia: a regional analysis of COMMODORE in China, South-East Asia, and Russia
- PMID: 40063243
- PMCID: PMC12031924
- DOI: 10.1007/s00277-025-06235-y
Gilteritinib versus salvage chemotherapy in predominantly Asian patients with relapsed/refractory FLT3-mutated acute myeloid leukemia: a regional analysis of COMMODORE in China, South-East Asia, and Russia
Abstract
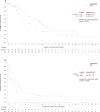
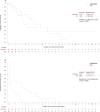
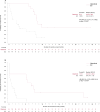
The COMMODORE study demonstrated the efficacy and safety of gilteritinib versus salvage chemotherapy (SC) treatment in a predominantly Asian population with relapsed/refractory (R/R) FMS-like tyrosine kinase 3 (FLT3)-mutated(mut+) acute myeloid leukemia (AML); here we present an exploratory analysis of the study stratified by region (China, South-East Asia and Russia). COMMODORE was a Phase 3, open-label, randomized (1:1), multicenter trial. There were 151, 50, and 33 patients in the China, South-East Asia, and Russia cohorts, respectively. Patients treated with gilteritinib had prolonged median overall survival (OS) versus SC-treated patients in all regions (China: 10.0 vs. 5.7 months, HR [95% CI]: 0.614 [0.385, 0.981]; South-East Asia: 7.8 vs. 4.7 months, HR [95% CI]: 0.887 [0.427, 1.843]; Russia: 8.8 vs. 2.6 months, HR [95% CI]: 0.271 [0.111, 0.662]). Improvements in event-free survival (EFS) were observed in the gilteritinib versus SC arms across all cohorts (China: 2.1 vs. 0.8 months; HR [95% CI]: 0.645 [0.427, 0.974]; South-East Asia 2.4 vs. < 0.1 months; HR [95% CI]: 0.415 [0.208, 0.830]; Russia: 6.2 vs. 0.6 months; HR [95% CI]: 0.221 [0.080, 0.614]). Complete remission rates were numerically higher in the gilteritinib versus SC arm across all three regions. Gilteritinib compared with SC treatment improved OS and EFS with no new safety signals, reinforcing the known efficacy and safety profile of gilteritinib in patients with R/R FLT3mut+ AML, and affirming the clinical benefit of gilteritinib in three different patient populations. ClinicalTrials.gov identifier: NCT03182244.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This trial was conducted in accordance with the study protocol, the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use guidelines and Declaration of Helsinki, and applicable regulations/guidelines governing clinical study conduct and ethical principles. Consent to participate: Written and signed informed consent was obtained from all patients or their guardian or legal representative prior to screening. The study sponsor ensured that the use and disclosure of protected health information obtained during the trial complied with federal and/or regional legislation related to the privacy and protection of personal information. Competing interests: JW, BJ, JL, LL, XD, HJ, JD, LG, SB, LWLL and EM have nothing to disclose. TS reports Astellas stocks. AK reports consulting and honoraria fees from Roche, Johnson & Johnson, Novartis, American Taiwan Biopharm, AstraZeneca, Dr. Reddy, Bristol Myers Squibb, and Apexcella; support for attending meetings and/or travel from American Taiwan Biopharm. XZ, TS, MK, YD, and NH are all employees of Astellas.
Figures
References
-
- Nagel G, Weber D, Fromm E, Erhardt S, Lübbert M, Fiedler W, Kindler T, Krauter J, Brossart P, Kündgen A, Salih HR, Westermann J, Wulf G, Hertenstein B, Wattad M, Götze K, Kraemer D, Heinicke T, Girschikofsky M, Derigs HG, Horst HA, Rudolph C, Heuser M, Göhring G, Teleanu V, Bullinger L, Thol F, Gaidzik VI, Paschka P, Döhner K, Ganser A, Döhner H, Schlenk RF (2017) Epidemiological, genetic, and clinical characterization by age of newly diagnosed acute myeloid leukemia based on an academic population-based registry study (AMLSG BiO). Ann Hematol 96(12):1993–2003. 10.1007/s00277-017-3150-3 - PMC - PubMed
-
- Schneider F, Hoster E, Schneider S, Dufour A, Benthaus T, Kakadia PM, Bohlander SK, Braess J, Heinecke A, Sauerland MC, Berdel WE, Buechner T, Woermann BJ, Feuring-Buske M, Buske C, Creutzig U, Thiede C, Zwaan MC, Van Den Heuvel-Eibrink MM, Reinhardt D, Hiddemann W, Spiekermann K (2012) Age-dependent frequencies of NPM1 mutations and FLT3-ITD in patients with normal karyotype AML (NK-AML). Ann Hematol 91(1):9–18. 10.1007/s00277-011-1280-6 - PubMed
-
- Sakaguchi M, Yamaguchi H, Kuboyama M, Najima Y, Usuki K, Ueki T, Oh I, Mori S, Kawata E, Uoshima N, Kobayashi Y, Kako S, Tajika K, Shono K, Kayamori K, Hagihara M, Kanda J, Uchiyama H, Kuroda J, Uchida N, Kubota Y, Kimura S, Kurosawa S, Date K, Nakajima N, Marumo A, Omori I, Fujiwara Y, Terada K, Yui S, Wakita S, Arai K, Kitano T, Kakihana K, Kanda Y, Ohashi K, Fukuda T, Inokuchi K (2019) Significance of FLT3-tyrosine kinase domain mutation as a prognostic factor for acute myeloid leukemia. Int J Hematol 110(5):566–574. 10.1007/s12185-019-02720-z - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

