Interfacing with the Brain: How Nanotechnology Can Contribute
- PMID: 40063703
- PMCID: PMC11948619
- DOI: 10.1021/acsnano.4c10525
Interfacing with the Brain: How Nanotechnology Can Contribute
Abstract
Interfacing artificial devices with the human brain is the central goal of neurotechnology. Yet, our imaginations are often limited by currently available paradigms and technologies. Suggestions for brain-machine interfaces have changed over time, along with the available technology. Mechanical levers and cable winches were used to move parts of the brain during the mechanical age. Sophisticated electronic wiring and remote control have arisen during the electronic age, ultimately leading to plug-and-play computer interfaces. Nonetheless, our brains are so complex that these visions, until recently, largely remained unreachable dreams. The general problem, thus far, is that most of our technology is mechanically and/or electrically engineered, whereas the brain is a living, dynamic entity. As a result, these worlds are difficult to interface with one another. Nanotechnology, which encompasses engineered solid-state objects and integrated circuits, excels at small length scales of single to a few hundred nanometers and, thus, matches the sizes of biomolecules, biomolecular assemblies, and parts of cells. Consequently, we envision nanomaterials and nanotools as opportunities to interface with the brain in alternative ways. Here, we review the existing literature on the use of nanotechnology in brain-machine interfaces and look forward in discussing perspectives and limitations based on the authors' expertise across a range of complementary disciplines─from neuroscience, engineering, physics, and chemistry to biology and medicine, computer science and mathematics, and social science and jurisprudence. We focus on nanotechnology but also include information from related fields when useful and complementary.
Keywords: Nanoneuro interface; brain-on-a-chip; brain−machine interfaces; control of ion channels; deep brain stimulation; electrode arrays; extracellular recordings; nanostructured interface; neuro-implants; neuronal communication.
Conflict of interest statement
The authors declare the following competing financial interest(s): A.M.A. and P.S.W. have filed U.S. and foreign patents related to neurotransmitter sensors and sensor arrays. E.S. has U.S. patents on nanoporous gold electrodes for multifunctional neural interfaces and for electrochemical biosensors. J.A.G. and K.K. declare that they are Co-Founders and hold interest in INBRAIN Neuroelectronics that has licensed parts of the graphene-based neural interface technology described. N.A.R. and M.P. have patent applications pending related to the preparation of 3D scaffolds for nerve growth. Other authors declare no conflicts of interest.
Figures










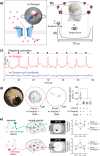
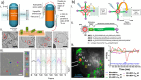

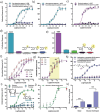

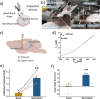











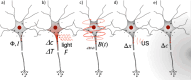

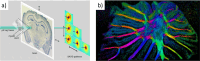
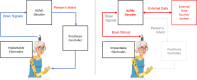
References
-
- Alivisatos A. P.; Andrews A. M.; Boyden E. S.; Chun M.; Church G. M.; Deisseroth K.; Donoghue J. P.; Fraser S. E.; Lippincott-Schwartz J.; Looger L. L.; Masmanidis S.; McEuen P. L.; Nurmikko A. V.; Park H.; Peterka D. S.; Reid C.; Roukes M. L.; Scherer A.; Schnitzer M.; Sejnowski T. J.; Shepard K. L.; Tsao D.; Turrigiano G.; Weiss P. S.; Xu C.; Yuste R.; Zhuang X. W. Nanotools for Neuroscience and Brain Activity Mapping. ACS Nano 2013, 7, 1850–1866. 10.1021/nn4012847. - DOI - PMC - PubMed
-
- Nuremberg Funnel. Wikipedia. https://en.wikipedia.org/wiki/Nuremberg_Funnel (accessed December 22, 2023).
-
- Orwell G.1984; Houghton Mifflin Harcour: New York, 1983.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

