Highly Specific Miniaturized Fluorescent Monoacylglycerol Lipase Probes Enable Translational Research
- PMID: 40063733
- PMCID: PMC11951083
- DOI: 10.1021/jacs.4c15223
Highly Specific Miniaturized Fluorescent Monoacylglycerol Lipase Probes Enable Translational Research
Abstract
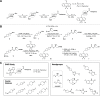
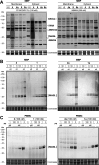
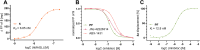
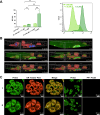
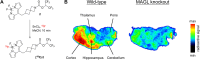
Monoacylglycerol lipase (MAGL) is the pivotal catabolic enzyme responsible for signal termination in the endocannabinoid system. Inhibition of MAGL offers unique advantages over the direct activation of cannabinoid receptors in treating cancer, metabolic disorders, and inflammatory diseases. Although specific fluorescent molecular imaging probes are commonly used for the real-time analysis of the localization and distribution of drug targets in cells, they are almost invariably composed of a linker connecting the pharmacophore with a large fluorophore. In this study, we have developed miniaturized fluorescent probes targeting MAGL by incorporating a highly fluorescent boron-dipyrromethene (BODIPY) moiety into the inhibitor structure that interacts with the MAGL active site. These miniaturized fluorescent probes exhibit favorable drug-like properties such as high solubility and permeability, picomolar potency for MAGL across various species, and high cell selectivity and specificity. A range of translational investigations were conducted, including cell-free fluorescence polarization assays, fluorescence-activated cell sorting analysis, and confocal fluorescence microscopy of live cancer cells, live primary neurons, and human-induced pluripotent stem cell-derived brain organoids. Furthermore, the application of red-shifted analogs or 18F positron emission labeling illustrated the significant versatility and adaptability of the fluorescent ligands in various experimental contexts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

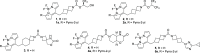


References
-
- Nomura D. K.; Morrison B. E.; Blankman J. L.; Long J. Z.; Kinsey S. G.; Marcondes M. C. G.; Ward A. M.; Hahn Y. K.; Lichtman A. H.; Conti B.; Cravatt B. F. Endocannabinoid Hydrolysis Generates Brain Prostaglandins That Promote Neuroinflammation. Science 2011, 334 (6057), 809–813. 10.1126/science.1209200. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

