Epstein-Barr virus and the immune microenvironment in multiple sclerosis: Insights from high-dimensional brain tissue imaging
- PMID: 40063794
- PMCID: PMC11929469
- DOI: 10.1073/pnas.2425670122
Epstein-Barr virus and the immune microenvironment in multiple sclerosis: Insights from high-dimensional brain tissue imaging
Abstract
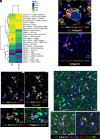
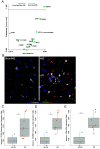
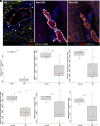
Epstein-Barr virus (EBV) is strongly implicated in the pathogenesis of multiple sclerosis (MS), yet its exact role in disease progression remains unclear. Using high-dimensional CO-detection by indexing, a technology for spatial imaging, this study examines the cellular microenvironment of MS lesions in secondary progressive MS and primary progressive MS. We analyzed immune, glial, neuronal, and endothelial cell interactions within MS lesions and normal-appearing white matter across two independent cohorts. Our findings show the enrichment of EBV markers, particularly EBNA1 and LMP1, within MS lesions. EBV-positive cells interact closely with reactive astrocytes, microglia, and neurons. Image analysis confirmed the presence of EBV-positive staining within neurons and glial cells, suggesting a direct role for EBV in neuronal and glial involvement in MS. Additionally, we observed altered immune cell interactions, including reduced associations with macrophages and memory T cells, and enhanced interactions with glial cells. Disruptions in blood-brain barrier integrity were also noted in regions of the MS brain. These results highlight EBV's contribution to immune modulation, glial dysfunction, and neuronal damage in MS, particularly in progressive subtypes. The analysis of MS brain tissue suggests potential therapeutic targets, including antivirals and brain penetrant immune modulators, to address EBV's impact on MS progression.
Keywords: Epstein–Barr virus; high-dimensional profiling; molecular mimicry; multiple sclerosis; tissue profiling.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

