Astrocytes in the mouse brain respond bilaterally to unilateral retinal neurodegeneration
- PMID: 40063795
- PMCID: PMC11929491
- DOI: 10.1073/pnas.2418249122
Astrocytes in the mouse brain respond bilaterally to unilateral retinal neurodegeneration
Abstract
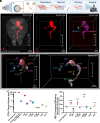
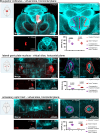
Glaucomatous optic neuropathy, or glaucoma, is the world's primary cause of irreversible blindness. Glaucoma is comorbid with other neurodegenerative diseases, but how it might impact the environment of the full central nervous system to increase neurodegenerative vulnerability is unknown. Two neurodegenerative events occur early in the optic nerve, the structural link between the retina and brain: loss of anterograde transport in retinal ganglion cell (RGC) axons and early alterations in astrocyte structure and function. Here, we used whole-mount tissue clearing of full mouse brains to image RGC anterograde transport function and astrocyte responses across retinorecipient regions early in a unilateral microbead occlusion model of glaucoma. Using light sheet imaging, we found that RGC projections terminating specifically in the accessory optic tract are the first to lose transport function. Although degeneration was induced in one retina, astrocytes in both brain hemispheres responded to transport loss in a retinotopic pattern that mirrored the degenerating RGCs. A subpopulation of these astrocytes in contact with large descending blood vessels were immunopositive for LCN2, a marker associated with astrocyte reactivity. Together, these data suggest that even early stages of unilateral glaucoma have broad impacts on the health of astrocytes across both hemispheres of the brain, implying a glial mechanism behind neurodegenerative comorbidity in glaucoma.
Keywords: astrocyte; glaucoma; neurodegeneration; reactivity; visual system.
Conflict of interest statement
Competing interests statement:S.A.L. maintains a financial interest in AstronauTx and Synapticure. S.A.L. is on the Scientific Advisory Board of the Global BioAccess Fund. S.A.L. and T.B. are co-authors on a review (PMID: 35313950).
Figures


Comment in
-
Bilateral astrocyte reaction to unilateral insult in the optic projection to the brain.Proc Natl Acad Sci U S A. 2025 Apr 8;122(14):e2502602122. doi: 10.1073/pnas.2502602122. Epub 2025 Mar 31. Proc Natl Acad Sci U S A. 2025. PMID: 40163738 Free PMC article. No abstract available.
References
-
- Tham Y. C., et al. , Global prevalence of glaucoma and projections of glaucoma burden through 2040: A systematic review and meta-analysis. Ophthalmology 121, 2081–2090 (2014). - PubMed
-
- Stein J. D., Khawaja A. P., Weizer J. S., Glaucoma in adults-screening, diagnosis, and management: A review. JAMA 325, 164–174 (2021). - PubMed
-
- Weinreb R. N., et al. , Risk assessment in the management of patients with ocular hypertension. Am. J. Ophthalmol. 138, 458–467 (2004). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

