Impact of mass drug administration with ivermectin, diethylcarbamazine, and albendazole for lymphatic filariasis on hookworm and Strongyloides stercoralis infections in Papua New Guinea
- PMID: 40063867
- PMCID: PMC11893124
- DOI: 10.1371/journal.pntd.0012851
Impact of mass drug administration with ivermectin, diethylcarbamazine, and albendazole for lymphatic filariasis on hookworm and Strongyloides stercoralis infections in Papua New Guinea
Abstract
Background: Persons with lymphatic filariasis (LF) are often co-infected with soil-transmitted helminths. A single co-administered dose of ivermectin/diethylcarbamazine/albendazole (IDA) is recommended by WHO for mass drug administration (MDA) for LF instead of diethylcarbamazine/albendazole (DA) in Papua New Guinea (PNG). We compared the effectiveness of a single round of MDA with IDA or DA on hookworm and strongyloidiasis in PNG.
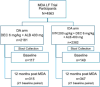
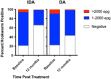
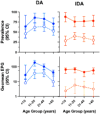
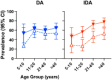
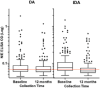
Methodology/principal findings: This study was conducted as part of a cluster randomized trial of MDA with IDA versus DA for LF in individuals willing to provide stool and blood samples at baseline and 12 months after MDA. Participants from 23 villages were included in the clinical trial. Primary outcomes were changes in hookworm prevalence and infection intensity assessed by Kato Katz and Strongyloides prevalence by serology. Hookworm prevalence at baseline was 78% (91/117) and 80% (119/149) in villages assigned to DA and IDA treatment, respectively. Twelve months post-MDA, hookworm prevalence decreased to 56.5% in DA- and 34.4% in IDA-treated villages, respectively (p<0.001, both comparisons). The proportion of individuals with moderate to heavy infection (>2000 egg per gram (EPG)) similarly decreased from 8.7% to 1.5% after DA (p = 0.001) and from 5.7% to 1.0% after IDA (p = 0.002). Using a logistic regression model adjusting for age, gender, baseline hookworm prevalence, and village drug coverage, IDA resulted in a 45% greater reduction in hookworm prevalence than DA (Odds ratio 0.55, 95% CI [0.31,0.99], p = 0.049). MDA also reduced hookworm transmission. Strongyloides seroprevalence at baseline was 68% (192/283) and 62% (180/290) in IDA and DA villages, respectively, with 49% becoming seronegative in the IDA versus 23% in DA villages at 12 months (p = 0.0001).
Conclusions/significance: MDA with IDA was more effective than DA for reducing hookworm and Strongyloides infections in PNG, extending the benefit of MDA with IDA beyond its effect on LF.
Copyright: This is an open access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Shield JM, Kow F. A comparative study of intestinal helminths in pre-school-age urban and rural children in Morobe Province, Papua New Guinea. P N G Med J. 2013;56(1–2):14–31. - PubMed
-
- Ashford R, Vince J, Gratten M, Bana-Koiri J. Strongyloides infection in a mid-mountain Papua New Guinea community: results of an epidemiological survey. P N G Med J. 2005;48(1–2):58–65. - PubMed
-
- Shield JM, Vaterlaws AL, Kimber RJ, Payne R, Casey GJ, Blunden RW, et al.. The relationship of hookworm infection, anaemia and iron status in a Papua New Guinea highland population and the response to treatment with iron and mebendazole. P N G Med J. 1981;24(1):19–34. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

