Prospective Analysis of urINe LAM to Eliminate NTM Sputum Screening (PAINLESS) study: Rationale and trial design for testing urine lipoarabinomannan as a marker of NTM lung infection in cystic fibrosis
- PMID: 40063876
- PMCID: PMC11893114
- DOI: 10.1371/journal.pone.0309191
Prospective Analysis of urINe LAM to Eliminate NTM Sputum Screening (PAINLESS) study: Rationale and trial design for testing urine lipoarabinomannan as a marker of NTM lung infection in cystic fibrosis
Abstract
Background: Routine screening for nontuberculous mycobacterial (NTM) lung disease is dependent on sputum cultures. This is particularly challenging in the cystic fibrosis (CF) population due to reduced sputum production and low culture sensitivity. Biomarkers of infection that do not rely on sputum may lead to earlier diagnosis, but validation trials require a unique prospective design.
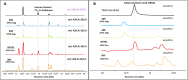
Purpose: The rationale of this trial is to investigate the utility of urine lipoarabinomannan (LAM) as a test to identify people with CF with a new positive NTM culture. We hypothesize that urine LAM is a sensitive, non-invasive screening test with a high negative predictive value to identify individuals with a relatively low risk of having positive NTM sputum culture.
Study design: This is a prospective, single-center, non-randomized observational study in adults with CF, 3 years of negative NTM cultures, and no known history of NTM positive cultures. Patients are followed for two year-long observational periods with the primary endpoint being a positive NTM sputum culture within a year of a positive urine LAM result and a secondary endpoint of a positive NTM sputum culture within 3 years of a positive urine LAM result. Study implementation includes remote consent and sample collection to accommodate changes from the COVID-19 pandemic.
Conclusions: This report describes the study design of an observational study aimed at using a urine biomarker to assist in the diagnosis of NTM lung infection in pwCF. If successful, urine LAM could be used as an adjunct to traditional sputum cultures for routine NTM screening, and replace cultures in low-risk individuals unable to produce sputum.
Copyright: © 2025 Calhoun et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Update of
-
Prospective Analysis of urINe LAM to Eliminate NTM Sputum Screening (PAINLESS) study: Rationale and trial design for testing urine lipoarabinomannan as a marker of NTM lung infection in cystic fibrosis.medRxiv [Preprint]. 2024 Aug 9:2024.08.08.24311698. doi: 10.1101/2024.08.08.24311698. medRxiv. 2024. Update in: PLoS One. 2025 Mar 10;20(3):e0309191. doi: 10.1371/journal.pone.0309191. PMID: 39148848 Free PMC article. Updated. Preprint.
References
-
- Floto RA, Olivier KN, Saiman L, Daley CL, Herrmann J-L, Nick JA, et al.. US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis. Thorax. 2016;71 Suppl 1(Suppl 1):i1–22. doi: 10.1136/thoraxjnl-2015-207360 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

