A real-world experience of efficacy and safety of belantamab mafodotin in relapsed refractory multiple myeloma
- PMID: 40064854
- PMCID: PMC11893888
- DOI: 10.1038/s41408-025-01226-8
A real-world experience of efficacy and safety of belantamab mafodotin in relapsed refractory multiple myeloma
Abstract
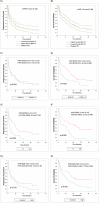
While initial trials led to the accelerated approval of belantamab mafodotin, a BCMA-directed antibody-drug conjugate, confirmatory trials failed to establish benefit from this therapy for patients with relapsed refractory multiple myeloma (RRMM), eventually leading to its withdrawal from commercial use. With an imminent approval as an effective combination therapy, as seen in recent randomized trials, we report real-world clinical outcomes with belantamab mafodotin in 81 RRMM patients. With a median of 5 (range 2-15) prior lines of therapy, 92, 45, and 15% of the patients were triple-class refractory, penta-class refractory, and BCMA-refractory. More than half (57%) of the patients had high-risk cytogenetics, 37% had extramedullary disease (EMD), and 67% of the patients would have been considered ineligible for the DREAMM-2 trial. The best overall response (ORR) and complete response rates were 40.0 and 15.0%, respectively. ORRs were lower in patients with EMD, BCMA-refractory, and penta-refractory disease at 23, 17, and 24%, respectively. All-grade ocular toxicity was seen in 69% of patients, with grade 3+ events in 43%. Grade 3+ hematological toxicities included neutropenia (20%), anemia (28%), and thrombocytopenia (31%). With a median follow-up of 11.3 (0.3-44.6) months for the entire population, median PFS and OS were 5 (1-20) months and 12 (3-28) months, respectively. Presence of EMD was the only predictor of both PFS and OS on multivariable analysis. Compared to the pivotal trial and despite several high-risk disease features, belantamab mafodotin demonstrated comparable efficacy and safety in this real-world patient population.
© 2025. The Author(s).
Conflict of interest statement
Conflict of interest: Authors RD, KB, YY, EC, HS, KG, OA, AR, BP, and A-OA have no conflicts of interest to disclose. HH disclosed Janssen, Amgen, Honoraria, and Karyopharm as conflicts of interest. PM disclosed Celegene and Pfizer as conflict of interests. CS has disclosed Pfizer, Janssen, Bristol Meyer Squibb, Seagen, and Poseida as conflict of interests. JD has disclosed Janssen Biotech as a conflict of interest. NA has disclosed BMS, Kite/Gilead, and Legend Biotech as conflicts of interest. Consent to participate: All methods were performed in accordance with the relevant guidelines and regulations. As this is a multicenter consortium study, the study was reviewed and approved by the institutional review boards (incorporating the ethics committee) at each site. As this is a multicenter consortium study utilizing retrospective data, the study was reviewed and approved by the institutional review boards (incorporating ethics committee) at each site, and the need for obtaining informed consent from the participants was waived. Ethical approval: This multicenter study was a collaborative effort by US Myeloma Innovations Research Collaborative (USMIRC) and was approved by respective institutions’ Institutional Review Board.
Figures
References
-
- Lonial S, Lee HC, Badros A, Trudel S, Nooka AK, Chari A, et al. Belantamab mafodotin for relapsed or refractory multiple myeloma (DREAMM-2): a two-arm, randomised, open-label, phase 2 study. Lancet Oncol. 2020;21:207–21. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

