Impact of liver fibrosis on AAV-mediated gene transfer to mouse hepatocytes
- PMID: 40064861
- PMCID: PMC11893804
- DOI: 10.1038/s41467-025-57382-9
Impact of liver fibrosis on AAV-mediated gene transfer to mouse hepatocytes
Abstract
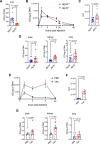
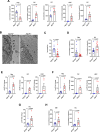
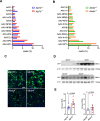
Liver fibrosis, characterized by scar tissue accumulation due to liver injury, poses significant barriers to liver-targeted gene therapy. Current clinical trials exclude patients with fibrosis, as intact liver architecture is considered essential for efficient and safe adeno-associated viral vector (AAV)-mediated gene delivery. Here, we show that liver fibrosis reduces the efficiency of hepatocyte transduction by AAV8 vectors across three mouse models with diverse fibrotic patterns. This inefficiency stems primarily from decreased vector uptake by the liver rather than loss of vector genomes due to hepatocyte turnover. Additionally, fibrosis alters blood vector clearance and redistributes AAV particles to extra-hepatic organs, such as spleen, lung, and kidney. At the cellular level, fibrosis decreases AAV genome content in hepatocytes while increasing it in non-parenchymal liver cells and splenic immune cells. Importantly, the capsid variant AAV-KP1 retains transduction efficiency in fibrotic livers, highlighting its potential for expanding gene therapy applications to fibrotic diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: R.F., N.B.P., and P.P. are inventors in patent n. WO2022184650 -Use of microRNAs in the treatment of fibrosis. The remaining authors declare no competing interests.
Figures


References
-
- Harris, R., Harman, D. J., Card, T. R., Aithal, G. P. & Guha, I. N. Prevalence of clinically significant liver disease within the general population, as defined by non-invasive markers of liver fibrosis: a systematic review. Lancet Gastroenterol. Hepatol.2, 288–297 (2017). - PubMed
-
- Ranucci, G. et al. Chronic liver involvement in urea cycle disorders. J. Inherit. Metab. Dis.42, 1118–1127 (2019). - PubMed
-
- Burrow, T. A., Bove, K. E. & Grabowski, G. A. The liver in lysosomal storage diseases. In Liver Disease in Children 3 edn (eds Suchy, F. J., Sokol, R. J. & Balistreri, W. F.) (Cambridge Univ. Press, 2007).
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

