In vivo prime editing rescues photoreceptor degeneration in nonsense mutant retinitis pigmentosa
- PMID: 40064881
- PMCID: PMC11893901
- DOI: 10.1038/s41467-025-57628-6
In vivo prime editing rescues photoreceptor degeneration in nonsense mutant retinitis pigmentosa
Abstract
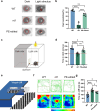
The next-generation gene editing tool, prime editing (PE), is adept at correcting point mutations precisely with high editing efficiency and rare off-target events and shows promising therapeutic value in treating hereditary diseases. Retinitis pigmentosa (RP) is the most common type of inherited retinal dystrophy and is characterized by progressive degeneration of retinal photoreceptors and, consequently, visual decline. To date, effective treatments for RP are lacking. Herein, a PE system is designed to target the PDE6B Y347X mutation in the rd1 mouse strain, a preclinical RP model. We screen and develop the PE system with epegRNA and RTΔRnH, which is delivered via dual-AAV in vivo with an editing efficiency of 26.47 ± 13.35%, with negligible off-target effects confirmed by AID-Seq and PE-tag. Treatment with the PE system in vivo greatly restores PDE6B protein expression and protects rod cells from degeneration. Mouse behavioural experiments also show that compared with no treatment, prime editing inhibits vision deterioration in littermate rd1 mice. This study provides a therapeutic opportunity for the use of PE to correct mutated RPs at the genomic level.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: D.R.L. is a co-founder and consultant for Prime Medicine, Beam Therapeutics, Pairwise Plants, and nChroma Bio, and owns equity in these companies. The other authors declare no competing interests.
Figures

References
-
- Fu, Y. et al. Prime editing: current advances and therapeutic opportunities in human diseases. Sci. Bull.68, 3278–3291 (2023). - PubMed
-
- Jang, H. et al. Application of prime editing to the correction of mutations and phenotypes in adult mice with liver and eye diseases. Nat Biomed Eng, 10.1038/s41551-021-00788-9 (2021). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

