Impact of adapted physical activity and diet counselling on health-related quality of life in women undergoing adjuvant breast cancer therapy
- PMID: 40064980
- PMCID: PMC11893781
- DOI: 10.1038/s41598-025-91569-w
Impact of adapted physical activity and diet counselling on health-related quality of life in women undergoing adjuvant breast cancer therapy
Abstract
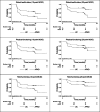
In the monocentric APAD1 trial, 143 women with non-metastatic breast cancer were randomised to undergo either an adapted physical activity and diet counselling (APAD) program or usual care. Health-related quality of life (HRQoL) was prospectively evaluated using the EORTC QLQ-C30 questionnaire at baseline, during treatment (adjuvant chemotherapy and radiotherapy) and during follow-up. Our objective was two-fold: to analyse the impact of APAD on HRQoL using three approaches; to illustrate the advantages and disadvantages of each approach, and derive methodological recommendations. Analytical approaches utilised were: statistical testing to compare the mean HRQoL scores between baseline and end of study in both groups and the mean HRQoL scores between the two groups at the different assessment times; linear mixed models that modelled the longitudinal score data in both groups and tested whether the score trajectories were different between the groups; a survival analysis comparing the time to deterioration of HRQoL between the groups using a minimal clinically important difference. This study shows a substantial clinical benefit of the APAD intervention on HRQoL, especially for global health status/HRQoL, functioning scales and the fatigue symptom scale. Furthermore, this study highlights the advantages and disadvantages of three standard approaches used to analyse HRQoL data.Trial registration: The APAD1 study was registered with ClinicalTrials.gov (number NCT01495650, date 20/12/2011).
Keywords: Breast cancer; Clinical trial; Health-related quality of life; Linear mixed models; Longitudinal data; Time to deterioration.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The APAD1 study received approval of all French institutional review boards. All participants provided written informed. Competing interests: The authors declare no competing interests.
Figures

References
-
- Hagen, K. B. et al. Fatigue, anxiety and depression overrule the role of oncological treatment in predicting self-reported health complaints in women with breast cancer compared to healthy controls. Breast28, 100–106. 10.1016/j.breast.2016.05.005 (2016). - PubMed
-
- Gupta, D., Lis, C. G. & Grutsch, J. F. The relationship between cancer-related fatigue and patient satisfaction with quality of life in cancer. J. Pain Symptom Manag.34, 40–47. 10.1016/j.jpainsymman.2006.10.012 (2007). - PubMed
-
- Stasi, R., Abriani, L., Beccaglia, P., Terzoli, E. & Amadori, S. Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer98, 1786–1801. 10.1002/cncr.11742 (2003). - PubMed
-
- Branstrom, R., Petersson, L. M., Saboonchi, F., Wennman-Larsen, A. & Alexanderson, K. Physical activity following a breast cancer diagnosis: implications for self-rated health and cancer-related symptoms. Eur. J. Oncol. Nurs.19, 680–685. 10.1016/j.ejon.2015.04.008 (2015). - PubMed

