Subcellular proteomics and iPSC modeling uncover reversible mechanisms of axonal pathology in Alzheimer's disease
- PMID: 40065072
- PMCID: PMC11922768
- DOI: 10.1038/s43587-025-00823-3
Subcellular proteomics and iPSC modeling uncover reversible mechanisms of axonal pathology in Alzheimer's disease
Abstract
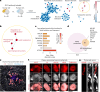
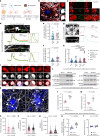
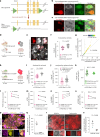
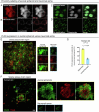
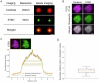
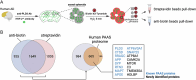
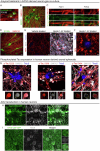
Dystrophic neurites (also termed axonal spheroids) are found around amyloid deposits in Alzheimer's disease (AD), where they impair axonal electrical conduction, disrupt neural circuits and correlate with AD severity. Despite their importance, the mechanisms underlying spheroid formation remain incompletely understood. To address this, we developed a proximity labeling approach to uncover the proteome of spheroids in human postmortem and mouse brains. Additionally, we established a human induced pluripotent stem cell (iPSC)-derived AD model enabling mechanistic investigation and optical electrophysiology. These complementary approaches revealed the subcellular molecular architecture of spheroids and identified abnormalities in key biological processes, including protein turnover, cytoskeleton dynamics and lipid transport. Notably, the PI3K/AKT/mTOR pathway, which regulates these processes, was activated in spheroids. Furthermore, phosphorylated mTOR levels in spheroids correlated with AD severity in humans. Notably, mTOR inhibition in iPSC-derived neurons and mice ameliorated spheroid pathology. Altogether, our study provides a multidisciplinary toolkit for investigating mechanisms and therapeutic targets for axonal pathology in neurodegeneration.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











References
-
- Tsai, J. et al. Fibrillar amyloid deposition leads to local synaptic abnormalities and breakage of neuronal branches. Nat. Neurosci.7, 1181–1183 (2004). - PubMed
-
- Adalbert, R. et al. Severely dystrophic axons at amyloid plaques remain continuous and connected to viable cell bodies. Brain132, 402–416 (2009). - PubMed
MeSH terms
Substances
Grants and funding
- Alzheimer's Association Research Fellowship (23AARF-1020552)/ALZ/Alzheimer's Association/United States
- BrightFocus Foundation Postdoctoral Fellowship Program/BrightFocus Foundation (BrightFocus)
- R01 DA018928/DA/NIDA NIH HHS/United States
- P30 AG072980/AG/NIA NIH HHS/United States
- P30 DA018343/DA/NIDA NIH HHS/United States
- R01NS111961/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- RF1 AG058257/AG/NIA NIH HHS/United States
- U24 NS072026/NS/NINDS NIH HHS/United States
- P30 AG019610/AG/NIA NIH HHS/United States
- RF1 AG065926/AG/NIA NIH HHS/United States
- R01 NS115544/NS/NINDS NIH HHS/United States
- R01NS115544/U.S. Department of Health & Human Services | NIH | National Institute of Neurological Disorders and Stroke (NINDS)
- S10 OD018034/OD/NIH HHS/United States
- EMBL-EBI Core funding/European Bioinformatics Institute (EMBL-EBI)
- P30 AG066508/AG/NIA NIH HHS/United States
- R01 AG068030/AG/NIA NIH HHS/United States
- R56 AG071291/AG/NIA NIH HHS/United States
- S10 OD019967/OD/NIH HHS/United States
- R01 NS111961/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

