Surface-mediated bacteriophage defense incurs fitness tradeoffs for interbacterial antagonism
- PMID: 40065098
- PMCID: PMC12048535
- DOI: 10.1038/s44318-025-00406-3
Surface-mediated bacteriophage defense incurs fitness tradeoffs for interbacterial antagonism
Abstract
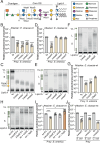
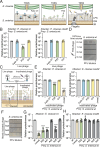
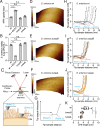
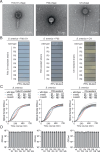
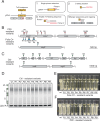
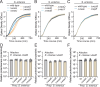
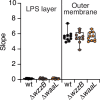
Bacteria in polymicrobial habitats are constantly exposed to biotic threats from bacteriophages (or "phages"), antagonistic bacteria, and predatory eukaryotes. These antagonistic interactions play crucial roles in shaping the evolution and physiology of bacteria. To survive, bacteria have evolved mechanisms to protect themselves from such attacks, but the fitness costs of resisting one threat and rendering bacteria susceptible to others remain unappreciated. Here, we examined the fitness consequences of phage resistance in Salmonella enterica, revealing that phage-resistant variants exhibited significant fitness loss upon co-culture with competitor bacteria. These phage-resistant strains display varying degrees of lipopolysaccharide (LPS) deficiency and increased susceptibility to contact-dependent interbacterial antagonism, such as the type VI secretion system (T6SS). Utilizing mutational analyses and atomic force microscopy, we show that the long-modal length O-antigen of LPS serves as a protective barrier against T6SS-mediated intoxication. Notably, this competitive disadvantage can also be triggered independently by phages possessing LPS-targeting endoglycosidase in their tail spike proteins, which actively cleave the O-antigen upon infection. Our findings reveal two distinct mechanisms of phage-mediated LPS modifications that modulate interbacterial competition, shedding light on the dynamic microbial interplay within mixed populations.
Keywords: Bacterial Immunity; Interbacterial Antagonism; Lipopolysaccharide; Phage; Tail Spike Protein.
© 2025. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures





References
-
- Aoki SK, Pamma R, Hernday AD, Bickham JE, Braaten BA, Low DA (2005) Contact-dependent inhibition of growth in Escherichia coli. Science 309:1245–1248 - PubMed
-
- Bastin DA, Stevenson G, Brown PK, Haase A, Reeves PR (1993) Repeat unit polysaccharides of bacteria: a model for polymerization resembling that of ribosomes and fatty acid synthetase, with a novel mechanism for determining chain length. Mol Microbiol 7:725–734 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

