Pelabresib plus ruxolitinib for JAK inhibitor-naive myelofibrosis: a randomized phase 3 trial
- PMID: 40065169
- PMCID: PMC12092244
- DOI: 10.1038/s41591-025-03572-3
Pelabresib plus ruxolitinib for JAK inhibitor-naive myelofibrosis: a randomized phase 3 trial
Abstract
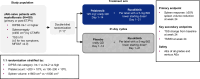
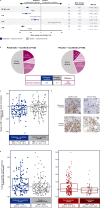
Janus kinase (JAK) inhibitors provide limited depth and durability of response in myelofibrosis. We evaluated pelabresib-a bromodomain and extraterminal domain (BET) inhibitor-plus ruxolitinib (a JAK inhibitor) compared with placebo plus ruxolitinib as first-line therapy. In this phase 3 study (MANIFEST-2), JAK inhibitor-naive patients with myelofibrosis were randomized 1:1 to pelabresib 125 mg once daily (QD; 50-175 mg QD permitted) for 14 days followed by a 7-day break (21-day cycle), or to placebo in combination with ruxolitinib 10 or 15 mg twice daily (BID; 5 mg QD-25 mg BID permitted). Primary endpoint was reduction in spleen volume of ≥35% from baseline at week 24. Key secondary endpoints were absolute change in total symptom score (TSS) and TSS50 response (≥50% reduction in TSS from baseline at week 24). The primary endpoint was met in 65.9% of patients randomized to pelabresib-ruxolitinib (n = 214) versus 35.2% to placebo-ruxolitinib (n = 216) (difference, 30.4%; 95% confidence interval (CI), 21.6, 39.3; P < 0.001). Absolute change in TSS was -15.99 versus -14.05 (difference, -1.94; 95% CI, -3.92, 0.04; P = 0.0545) and TSS50 was achieved in 52.3% versus 46.3% (difference, 6.0%; 95 CI, -3.5, 15.5) with pelabresib-ruxolitinib versus placebo-ruxolitinib. Exploratory analyses of proinflammatory cytokine amounts and bone marrow morphology showed greater improvement with the combination. Thrombocytopenia and anemia were the most common treatment-emergent adverse events, occurring in 52.8% (13.2% grade ≥3) versus 37.4% (6.1% grade ≥3) and 44.8% (23.1% grade ≥3) versus 55.1% (36.5% grade ≥3), respectively. Pelabresib in combination with ruxolitinib is well tolerated, improves signs of underlying myelofibrosis pathobiology and provides substantial clinical benefit over standard-of-care JAK inhibitor monotherapy. ClinicalTrials.gov identifier: NCT04603495 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors report the following competing interests. R.K.R. has received grants from MorphoSys/Constellation, Ryvu, Stemline and Zentalis, consultation fees from GlaxoSmithKline, Incyte, AbbVie, BMS-Celgene, Novartis, Zentalis, Promedior, CTI, Blueprint, Stemline, Galectco, PharmEssentia, Disc Medicines, Sunimoto Dainippon, Servier, Karyopharm and Cogent Bio, honoraria fees from Karyopharm, Sunimoto Dainippon and Protagonist, participated on a Data Safety Monitoring Board or Advisory Board for Disc Medicines and Merck, and received advisory fees from the MPN Research Foundation. D.C. has received consultation fees from Janssen, honoraria fees from Astellas, Amgen, BMS, Janssen, AstraZeneca and AbbVie, and support for attending meetings and/or travel from Gilead Sciences, Ryvu Therapeutics and AbbVie. E.A. has received consulting/advisory board fees from Ascentage, BMS, GlaxoSmithKline, Incyte, MorphoSys/Novartis, Pfizer and Takeda. P.B. has received research support from MorphoSys to his institution for the conduct of this clinical trial, institutional grants from Incyte, BMS, CTI BioPharma (a Sobi company), MorphoSys, Kartos, Telios, Karyopharm, Sumitomo, Janssen, Geron, Ionis, Disc, Blueprint and Cogent; consultation fees from Incyte, BMS, CTI BioPharma (a Sobi company), GlaxoSmithKline, AbbVie, MorphoSys, Karyopharm, Sumitomo, PharmaEssentia, Morphic, Jubilant, Ionis, Disc, Blueprint, Cogent, Ono and Novartis; honoraria fees from Incyte, Sumitomo, GlaxoSmithKline, PharmaEssentia, AbbVie, CTI BioPharma (a Sobi company) and Novartis; holds a study steering committee membership with Blueprint, Geron and Karyopharm, a steering committee membership with Sumitomo, Keros, GlaxoSmithKline and Karyopharm and a leadership role in a scientific advisory board with PharmaEssentia; is an advisory board member for Raythera; and had received MD Anderson Cancer Center Support grant P30 CA016672 from the National Institutes of Health (National Cancer Institute). A.T.G. has received consulting fees from Novartis (MorphoSys), PharmaEssentia, GlaxoSmithKline (Sierra Oncology), Sobi (CTI Biopharma), AbbVie, Merck (Imago Biosciences), Kartos, Telios, BMS, Rain Oncology, Disc Medicine and Agios. A.M.V. has received honoraria fees from Incyte, Novartis, AbbVie, GlaxoSmithKline, BMS, MorphoSys and AOP, participated on a Data Safety Monitoring Board or Advisory Board from Incyte, Novartis, AOP, MorphoSys and Roche. F.P. has received consultation and honoraria fees from Novartis, Celgene, AOP, Sierra Oncology and CTI. S.-E.L. declares no personal or financial conflicts of interests related to this manuscript, has received consulting/advisory board fees from BMS, GSK, Novartis and PharmaEssentia, and received an institutional grant from PharmaEssentia. V.G. has received grants from AbbVie and Novartis, consultation fees from GlaxoSmithKline, Incyte, AbbVie, BMS-Celgene and Novartis, honoraria fees from GlaxoSmithKline, Novartis and AbbVie, support for attending meetings and/or travel from GlaxoSmithKline and participated on a Data Safety Monitoring Board or Advisory Board from BMS-Celgene, Incyte, Daichii-Sankyo, AbbVie, Novartis and GlaxoSmithKline. A.L. has received consulting fees from AOP and Sanofi, honoraria fees from Grifols, Incyte, Novartis, Amgen, Pfizer, BMS, Sanofi and SOBI, support for attending meetings and/or travel from Sanofi and BeiGene, and participated on a Data Safety Monitoring Board or Advisory Board for MorphoSys, Amgen, Protagonist, Grifols, SOBI, Novartis and Sanofi. S.T.O. has received consulting fees from Novartis, Kartos Therapeutics, Disc Medicine, Blueprint Medicines, AbbVie, Constellation/MorphoSys, CTI BioPharma, Bristol Myers Squibb, Geron, GlaxoSmithKline/Sierra Oncology, Cogent and Incyte. A.T.K. has received institutional grants from MorphoSys, Novartis, BMS, GlaxoSmithKline, Protagonist, Geron and Janssen, consultation fees from AbbVie, MorphoSys and Karyopharm, honoraria fees from PharmaEssentia, Incyte, BMS and CTI Biopharma, support for attending meetings and/or travel support from PharmaEssentia, and participated on a Data Safety Monitoring Board or Advisory Board with Incyte. A.P. has received consulting fees from Sanofi and Sobi, honoraria fees from Sobi, Sanofi, Pfizer, Incyte, Alexion, Takeda, Novartis and BMS, and support for attending meetings and/or travel from Alexion, Sobi and Sanofi. A.A.-L. received honoraria fees from AOP Health for participating in an advisory board and from Novartis and GSK for lectures. R.M. has received grants for research support from MorphoSys, CTI, BMS, Genentech, AbbVie, Incyte and GlaxoSmithKline, and consultation fees from MorphoSys, Incyte, BMS, Novartis, CTI and GlaxoSmithKline. J.-J.K. has received consultation fees from Novartis, AbbVie and GlaxoSmithKline, honoraria fees from Novartis, AOP Health and BMS, support for attending meetings and/or travel from Novartis, and participated on a Data Safety Monitoring Board or Advisory Board from Incyte. M.T. has received advisory board fees from Novartis and Sumitomo, and research funding from BMS. J.M.S. received institutional research support from Constellation/MorphoSys, advisory board fees from Constellation/MorphoSys, grants from AbbVie (for research support, research support to institution, advisory board), SDP Oncology (for research support, research support to institution, advisory board), Karyopharm (for research support to institution, advisory board), Morphic (for research support), PharmaEssentia (for research support to institution, advisory board) and Protagonist (for research support to institution, advisory board), and consultation fees from SDP Oncology, Morphic, PharmaEssentia and Calico. D.L. has received consultation fees from AbbVie, Takeda and Novartis, honoraria fees from AbbVie, Roche, Novartis and Takeda, support for attending meetings and/or travel from AbbVie and Roche, and participated on a Data Safety Monitoring Board or Advisory Board from AbbVie. M.H. is an employee of Constellation Pharmaceuticals, a Novartis Company. S.-K.K. is an employee of MorphoSys GmbH, Planegg, Germany, a Novartis Company. A.-M.J. is an employee of MorphoSys GmbH, Planegg, Germany, a Novartis Company. Q.L. is an employee of MorphoSys US Inc., Boston, MA, USA, a Novartis Company. R.B. is an employee of MorphoSys GmbH, Planegg, Germany, a Novartis Company. B.B. was an employee of Constellation Pharmaceuticals, Boston, MA, USA, a Novartis Company, and holds stock with Pfizer, Moderna, Eli Lily, Exelixis and Verastem. C.N.H. has received institutional grants from Constellation and Novartis, consultation fees from Novartis, MSD, Karyopharm, AOP, GlaxoSmithKline, BMS, Sobi, Galecto and CTI, honoraria fees from Novartis, MSD, Karyopharm, Sobi, GlaxoSmithKline and BMS, support for attending meetings and/or travel from Novartis, participated on a Data Safety Monitoring Board or Advisory Board for BMS and Galecto, leadership role with Blood Cancer UK (Trustee; unpaid), EHA (Deputy Editor-in-Chief remunerated) and MPN Voice (Medical Director; unpaid), and holds stock or stock options with Chakana Medical Limited. J.M. has received grants from Incyte, Novartis, Geron, BMS, AbbVie, CTI/SOBI, Karyopharm, Disc Medicines, Ajax, PharmaEssentia and Kartos, consultation fees from Incyte, CTI/SOBI, BMS, MorphoSys, GlaxoSmithKline, AbbVie, Novartis, Roche, Merck, Pfizer, Geron, Karyopharm, PharmaEssentia, Disc Medicines, Blueprint Medicines, Keros, Galecto and Sumitomo, support for attending meetings and/or travel from Kartos, and participated on a Data Safety Monitoring or Advisory Board with Galecto and Incyte. The remaining author(s) declare no competing interests.
Figures








References
-
- Yacoub, A., Twardowski, N., Britt, A. & Shraim, N. SOHO state of the art updates and next questions | early intervention in myelofibrosis: where are we and does it matter? Clin. Lymphoma Myeloma Leuk.24, 506–511 (2024). - PubMed
-
- Tefferi, A. Primary myelofibrosis: 2023 update on diagnosis, risk‐stratification, and management. Am. J. Hematol.98, 801–821 (2023). - PubMed
-
- Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood127, 2391–2405 (2016). - PubMed
-
- Ng, Z. Y., Fuller, K. A., Mazza‐Parton, A. & Erber, W. N. Morphology of myeloproliferative neoplasms. Int. J. Lab. Hematol.45, 59–70 (2023). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

