LILRB3 genetic variation is associated with kidney transplant failure in African American recipients
- PMID: 40065170
- PMCID: PMC12527113
- DOI: 10.1038/s41591-025-03568-z
LILRB3 genetic variation is associated with kidney transplant failure in African American recipients
Erratum in
-
Author Correction: LILRB3 genetic variation is associated with kidney transplant failure in African American recipients.Nat Med. 2025 May;31(5):1712. doi: 10.1038/s41591-025-03706-7. Nat Med. 2025. PMID: 40234733 No abstract available.
Abstract
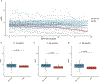
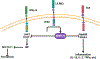
African American (AA) kidney transplant recipients exhibit a higher rate of graft loss compared with other racial and ethnic populations, highlighting the need to identify causative factors. Here, in the Genomics of Chronic Allograft Rejection cohort, pretransplant blood RNA sequencing revealed a cluster of four consecutive missense single-nucelotide polymorphisms (SNPs), within the leukocyte immunoglobulin-like receptor B3 (LILRB3) gene, strongly associated with death-censored graft loss. This SNP cluster (named LILRB3-4SNPs) encodes missense mutations at amino acids 617-618 proximal to a SHP1/2 phosphatase-binding immunoreceptor tyrosine-based inhibitory motif. The LILRB3-4SNPs cluster is specifically enriched within AA individuals and exhibited a strong association with death-censored graft loss and estimated glomerular filtration rate decline in the AA participants from multiple transplant cohorts. In two large Biobanks (BioMe and All-of-Us), the LILRB3-4SNPs cluster was associated with the early onset of end-stage renal disease and acted synergistically with the apolipoprotein L1 (APOL1) G1/G2 allele to accelerate disease progression. The SNPs were also linked to multiple immune-related diseases in AA individuals. Last, on multiomics analysis of blood and biopsies, recipients with LILRB3-4SNPs showed enhanced inflammation and monocyte ferroptosis. While larger and prospective studies are needed, our data provide insights on the genetic variation underlying kidney transplant outcomes.
© 2025. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests: Weijia Zhang reports personal fees from VericiDx and reports the patents (Patents US Provisional Patent Application F&R ref. 27527-0134P01, serial no. 61/951,651, filed March 2014; Method for identifying kidney allograft recipients at risk for chronic injury; US Provisional Patent Application: Methods for Diagnosing Risk of Renal Allograft Fibrosis and Rejection (miRNA); US Provisional Patent Application: Method for Diagnosing Subclinical Acute Rejection by RNA Sequencing Analysis of a Predictive Gene Set; US Provisional Patent Application: Pretransplant prediction of post- transplant acute rejection). M.C.M. receives research support from Natera. P. Cravedi is a consultant for Chinook therapeutics. L.G. is the non-executive Director and Chair of the science advisory board for Verici. The other investigators have no financial interest to declare.
Figures




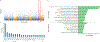




References
-
- Chertow GM & Milford EL Poorer graft survival in African-American transplant recipients cannot be explained by HLA mismatching. Adv Ren Replace Ther 4, 40–45 (1997). - PubMed
-
- Ng FL, Holt DW, Chang RW & Macphee IA Black renal transplant recipients have poorer long-term graft survival than CYP3A5 expressers from other ethnic groups. Nephrol Dial Transplant 25, 628–634 (2010). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

