Allogeneic mesenchymal stem cell therapy with laromestrocel in mild Alzheimer's disease: a randomized controlled phase 2a trial
- PMID: 40065171
- PMCID: PMC12003194
- DOI: 10.1038/s41591-025-03559-0
Allogeneic mesenchymal stem cell therapy with laromestrocel in mild Alzheimer's disease: a randomized controlled phase 2a trial
Abstract
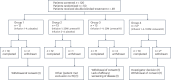
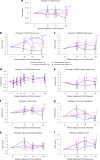
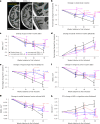
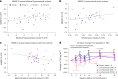
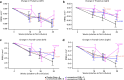
Alzheimer's disease (AD) is characterized by progressive cognitive decline, severe brain atrophy and neuroinflammation. We conducted a randomized, double-blind, placebo-controlled, parallel-group phase 2a clinical trial that tested the safety and efficacy of laromestrocel, a bone-marrow-derived, allogeneic mesenchymal stem-cell therapy, in slowing AD clinical progression, atrophy and neuroinflammation. Participants across ten centers in the United States were randomly assigned 1:1:1:1 to four infusion groups: group 1 (placebo; four monthly infusions, n = 12); group 2 (25 million cells, one infusion followed by three monthly infusions of placebo, n = 13); group 3 (25 million cells; four monthly doses, n = 13); and group 4 (100 million cells; four monthly doses, n = 11). The study met its primary end point of safety; the rate of treatment-emergent serious adverse events within 4 weeks of any infusion was similar in all four groups: group 1, 0% (95% CI 0-26.5%); group 2, 7.7% (95% CI 0.2-36%); group 3, 7.7% (95% CI 0.2-36%) and group 4, 9.1% (95% CI 0.2-41.3%). Additionally, there were no reported infusion-related reactions, hypersensitivities or amyloid-related imaging abnormalities. Laromestrocel improved clinical assessments at 39 weeks compared to placebo, as measured by a composite AD score (secondary end point was met: group 2 versus placebo change: 0.38; 95% CI -0.06-0.82), Montreal cognitive assessment and the Alzheimer's Disease Cooperative Study Activities of Daily Living. At 39 weeks, Laromestrocel slowed the decline of whole brain volume compared to placebo (n = 10) by 48.4% for all treatment groups combined (groups 2-4: P = 0.005; n = 32) and left hippocampal volume by 61.9% (groups 2-4, P = 0.021; n = 32), and reduced neuroinflammation as measured by diffusion tensor imaging. The change in bilateral hippocampal atrophy correlated with the change in mini-mental state exam scores (R = 0.41, P = 0.0075) in all study patients (N = 42). Collectively these results support safety of single and multiple doses of laromestrocel treatment for mild AD and provide indications of efficacy in combating decline of brain volume and potentially cognitive function. Larger-scale clinical trials of laromestrocel in AD are warranted. ClinicalTrials.gov registration: NCT05233774 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: J.M.H. reports having a patent for cardiac cell-based therapy and holds equity in Vestion and maintains a professional relationship with Vestion as a consultant and member of the Board of Directors and Scientific Advisory Board. Vestion did not play a role in the design, conduct, or funding of the study. J.M.H. is the Chief Scientific Officer, a compensated consultant and board member for Longeveron and holds equity in Longeveron. J.M.H. is also the co-inventor of intellectual property licensed to Longeveron. The University of Miami is an equity owner in Longeveron, which has licensed intellectual property from the University of Miami. B.G.R., N.A., L.M.M., Z.Z., B.V., K.P., M.B. and T.L. are active employees of Longeveron. The other authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

