Trajectories of pharmacological therapies for treatment-resistant depression: a longitudinal study
- PMID: 40065240
- PMCID: PMC11892204
- DOI: 10.1186/s12888-025-06518-8
Trajectories of pharmacological therapies for treatment-resistant depression: a longitudinal study
Abstract
Background: Treatment-resistant depression (TRD) in major depressive disorder (MDD) is defined as the failure of two or more antidepressants. Few studies have characterized the natural history and treatment patterns of these patients. This study aims to identify the natural history of disease and treatment trajectories for patients with TRD.
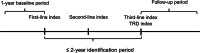
Methods: A retrospective longitudinal study used claims data linked to electronic health records (EHRs) from January 1, 2017, to October 31, 2021. Inclusion criteria were age ≥ 18 years, ≥ 1 MDD diagnosis, no antidepressant use at baseline, and an index date within 90 days of MDD diagnosis. Exclusions included psychiatric disorders other than MDD. TRD patients were defined as receiving third-line antidepressant treatment within two years of first-line initiation. Second- and third-line antidepressant treatment was defined as a switch to or addition of a different antidepressant with an adequate dose/duration or initiation of an augmentation treatment.
Results: Out of 301,821 individuals with MDD using antidepressants or augmentation medications during the study, 2,409 incident TRD patients were selected out of 50,374 meeting the criteria. The median time to TRD (time from first to third line index date) was 11.5 months, and the TRD episode duration was 10.8 months. Initial treatment was predominantly antidepressant monotherapy, declining from 91.0% in the first line to 39.4% in the third line. Combination therapy including antidepressants and augmentation medications increased over lines, reaching 55.6% in the third line. During the TRD episode, SSRIs were the most prescribed antidepressants with the longest duration of use. Cognitive-behavioral therapy was used by 53.5% of TRD patients, while other nonpharmacological therapies were rarely used. Treatment trajectories varied by age, sex, and anxiety.
Conclusions: This study identified contemporary treatment patterns in TRD patients, with combination therapy and augmentation medications increasingly used, highlighting the need for precision treatment based on individual trajectories.
Keywords: Antidepressant; Longitudinal study; Major depression disorder; Treatment-resistant depression.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study only involved the analysis of de-identified secondary data and did not directly involve human participants, human data, or human tissue. Therefore, it was exempt from review by the University of South Carolina Institutional Review Board. The use of de-identified secondary data did not require direct human participation or consent in accordance with HIPAA guidelines. Consent for publication: Not Applicable. All data used in this study analysis were de-identified. This study did not involve the collection of personal or clinical details from participants, nor does it present identifying images or data. Competing interests: Julia R. DiBello, Xinyue Liu, Wenjun Zhong, and Aristide Merola are current employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA and may hold stocks of Merck & Co., Inc., Rahway, NJ, USA.
Figures


References
-
- Association AP. What is depression? American Psychiatric Association. https://www.psychiatry.org/patients-families/depression/what-is-depression. Accessed 11 Oct 2023.
-
- Fava M. Diagnosis and definition of treatment-resistant depression. Biol Psychiatry. 2003;53(8):649–59. 10.1016/s0006-3223(03)00231-2. - PubMed
-
- Heerlein K, Perugi G, Otte C, et al. Real-world evidence from a European cohort study of patients with treatment resistant depression: treatment patterns and clinical outcomes. J Affect Disord. 2021;290:334–44. 10.1016/j.jad.2021.03.073. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

