Extraction of a stromal metastatic gene signature in breast cancer via spatial profiling
- PMID: 40065328
- PMCID: PMC11892262
- DOI: 10.1186/s13046-025-03353-3
Extraction of a stromal metastatic gene signature in breast cancer via spatial profiling
Abstract
Background: The identification of molecular features characterizing metastatic disease is a critical area of oncology research, as metastatic foci often exhibit distinct biological behaviors compared to primary tumors. While the focus has largely been on the neoplastic cells themselves, the characterization of the associated stroma remains largely underexplored, with significant implications for understanding metastasis.
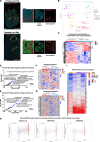
Main body: By employing spatially resolved transcriptomics, we analyzed the transcriptional features of primary breast adenocarcinoma and its associated metastatic foci, on a representative set of microregions. We identified a stromal metastatic (Met) signature, which was subsequently validated across transcriptomic reference human breast cancer (BC) datasets and in spatial transcriptomics of a murine model.
Conclusion: We discuss the potential of a stromal Met signature to pinpoint metastatic breast cancer, serving as a prognostic tool that can provide a foundation for the exploration of tumor-extrinsic molecular hallmarks of BC metastatic foci.
Keywords: Metastatic disease; Prognostic biomarker; Spatial transcriptomics; Stromal microenvironment.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study has been approved by the University Hospital of Palermo Ethical Review Board (approval number 06/2021) and by ethics committee for clinical trials of the IOV (approval number CESC IOV: 2022/125). Consent for publication: All authors agree with the content of the manuscript. Competing interests: The authors declare no competing financial interests.
Figures

References
-
- Peinado H, et al. Pre-metastatic niches: organ-specific homes for metastases. Nat Rev Cancer. 2017;17:302–17. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

