Epidemiology of idiopathic pulmonary fibrosis in central and Western Pennsylvania
- PMID: 40065350
- PMCID: PMC11895235
- DOI: 10.1186/s12931-025-03164-2
Epidemiology of idiopathic pulmonary fibrosis in central and Western Pennsylvania
Abstract
Background/rationale: Idiopathic Pulmonary Fibrosis (IPF) is a chronic, progressive disease of unknown origin. Establishing the epidemiology of IPF has been challenging due to diagnostic complexity, poor survival, low prevalence, and heterogeneity of ascertainment methodologies.
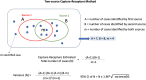
Objectives: This research aimed to estimate the rates of IPF in central and western Pennsylvania and to pilot the use of capture recapture (CR) methods to estimate the disease incidence.
Methods: We identified adults ≥ 30 years old diagnosed with IPF (by ICD-9/10 coding) between 2013 to 2021 from two health systems (UPMC Health System and Penn State Health) participating in the PaTH Clinical Research Network. We extracted information on patients' sex, race, date of birth and 3-digit zip code from electronic health records (EHR). Incidence rate of IPF among Pennsylvania residents was calculated using three case definitions (broad and two restricted) and piloted the use of CR in estimating IPF incidence.
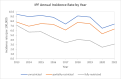
Results: IPF incidence rates were 8.42, 6.95 and 4.4 per 100,000 person-years for the unrestricted (n = 3148), partially restricted (n = 2598) and fully restricted (n = 1661) samples, respectively. Low case overlap between two sites resulted in a highly inflated estimate of IPF incidence, using the CR methodology.
Conclusions: The rate of IPF in central and western Pennsylvania was similar to previously published statistics. The application of CR to IPF epidemiology could be further investigated in health systems with greater overlap of patients utilizing more than one system.
Keywords: Epidemiology; Interstitial lung disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: University of Pittsburgh IRB # STUDY19080231 (exempt as non-human subject research). Penn State University IRB #STUDY00017636 (exempt as non-human subject research). Consent for publication: Not applicable. Competing interests: This was an independent, investigator initiated study supported by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI had no role in the design, analysis or interpretation of the results in this study; BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as it relates to BIPI substances, as well as intellectual property consideration.
Figures
References
MeSH terms
LinkOut - more resources
Full Text Sources