Trans-ancestral rare variant association study with machine learning-based phenotyping for metabolic dysfunction-associated steatotic liver disease
- PMID: 40065360
- PMCID: PMC11892324
- DOI: 10.1186/s13059-025-03518-5
Trans-ancestral rare variant association study with machine learning-based phenotyping for metabolic dysfunction-associated steatotic liver disease
Abstract
Background: Genome-wide association studies (GWAS) have identified common variants associated with metabolic dysfunction-associated steatotic liver disease (MASLD). However, rare coding variant studies have been limited by phenotyping challenges and small sample sizes. We test associations of rare and ultra-rare coding variants with proton density fat fraction (PDFF) and MASLD case-control status in 736,010 participants of diverse ancestries from the UK Biobank, All of Us, and BioMe and performed a trans-ancestral meta-analysis. We then developed models to accurately predict PDFF and MASLD status in the UK Biobank and tested associations with these predicted phenotypes to increase statistical power.
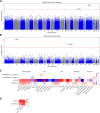
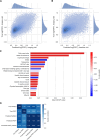
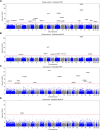
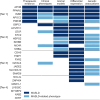
Results: The trans-ancestral meta-analysis with PDFF and MASLD case-control status identifies two single variants and two gene-level associations in APOB, CDH5, MYCBP2, and XAB2. Association testing with predicted phenotypes, which replicates more known genetic variants from GWAS than true phenotypes, identifies 16 single variants and 11 gene-level associations implicating 23 additional genes. Two variants were polymorphic only among African ancestry participants and several associations showed significant heterogeneity in ancestry and sex-stratified analyses. In total, we identified 27 genes, of which 3 are monogenic causes of steatosis (APOB, G6PC1, PPARG), 4 were previously associated with MASLD (APOB, APOC3, INSR, PPARG), and 23 had supporting clinical, experimental, and/or genetic evidence.
Conclusions: Our results suggest that trans-ancestral association analyses can identify ancestry-specific rare and ultra-rare coding variants in MASLD pathogenesis. Furthermore, we demonstrate the utility of machine learning in genetic investigations of difficult-to-phenotype diseases in trans-ancestral biobanks.
Keywords: Genetic association studies; Machine learning; Metabolic dysfunction-associated steatotic liver disease.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: In all three biobanks accessed in this study (UK Biobank, All of Us, BioMe), participants voluntarily enrolled and gave informed electronic consent. We accessed UK Biobank data under application ID 16218 and All of Us data (Controlled Tier version 7) under workspace aou-rw-75979bcb. The Institutional Review Board at the Icahn School of Medicine at Mount Sinai approved BioMe access (GCO no. 07–0529; STUDY-11–01139). Competing interests: R.D. reported being a scientific co-founder, consultant and equity holder for Pensieve Health (pending) and being a consultant for Variant Bio and Character Bio. M.B. receives grant support from Pfizer and Histoindex and serves as a consultant for Madrigal, Intercept, Fibronostics, NOVONordisk, GSK, and The Kinetix Group. All other authors have no competing interests to disclose.
Figures


References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

