Substantial Reduction of Systemic Corticosteroid Use After Primary Ileocaecal Resection in Swedish Patients With Crohn's Disease: A Population-Based Cohort Study
- PMID: 40065562
- PMCID: PMC12013787
- DOI: 10.1111/apt.70069
Substantial Reduction of Systemic Corticosteroid Use After Primary Ileocaecal Resection in Swedish Patients With Crohn's Disease: A Population-Based Cohort Study
Abstract
Background: The corticosteroid-sparing effects of ileocaecal resection have not been thoroughly investigated in a population-based cohort.
Aim: To investigate systemic corticosteroid use before and after primary ileocaecal resection in patients with Crohn's disease.
Methods: Through nationwide registries, we identified 1565 patients with Crohn's disease undergoing primary ileocaecal resection in Sweden 2006-2019. We stratified patients according to mean annual systemic corticosteroid (prednisolone equivalents) use in the last 5 years before surgery and compared Crohn's disease treatment after surgery.
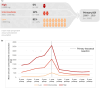
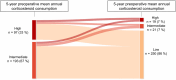
Results: Some 19% (290/1565) of the patients had a mean annual corticosteroid use of ≥ 1000 mg up to 5 years pre-operatively, of whom 33% (97/290) had ≥ 2000 mg. Mean annual pre-operative CS use did not decrease during the study period (p = 0.35). Compared with patients with < 1000 mg/year pre-operative steroid use, patients with ≥ 1000 mg/year had more frequent previous bowel surgery (10% vs. 16%), exposure to biologics (29% vs. 38%), and immunomodulators (56% vs. 83%). Patients with a pre-operative mean annual corticosteroid use of ≥ 1000 mg had a mean annual reduction in corticosteroid use of 1354 mg after ileocaecal resection (1847 mg pre-operative versus 493 mg post-operative). During follow-up (median 6.8 years), exposure to biologics was similar among patients with different levels of pre-operative corticosteroid use.
Conclusion: Our results suggest a significant corticosteroid-sparing effect of ileocaecal resection in Crohn's disease patients with high pre-operative use, indicating a beneficial outcome of earlier surgical intervention. Despite increasing use of biologics, pre-operative corticosteroid use was consistent over the study period.
Keywords: Crohn's disease; bowel surgery; corticosteroids; ileocaecal resection.
© 2025 The Author(s). Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Conflict of interest statement
Vilhelm Hjälte, Martin Rejler, Marcus Bendtsen and Åsa H. Everhov: these authors declare no conflicts of interest. Pär Myrelid: Myrelid has received honoraria for lectures from Ferring, AbbVie and Takeda and has served as an external consultant to Janssen and AbbVie and has been PI for a research project partly funded by Takeda. Henrik Hjortswang: Hjortswang has served as a speaker and advisory board member for Abbvie, Fresenius Kabi, Janssen, Norgine, Pfizer, Pharmacosmos, Takeda and Tillots Pharma. Jonas F. Ludvigsson: Ludvigsson has coordinated an unrelated study on behalf of the Swedish IBD quality register (SWIBREG). That study received funding from Janssen Corporation. Ludvigsson has also received financial support from MSD for developing a paper reviewing national healthcare registers in China. Ludvigsson has an ongoing research collaboration on celiac disease with Takeda. Anders Forss: Forss has served as a speaker and advisory board member for Janssen Cilag AB and Tillotts Pharma. Ola Olén: Olén has been PI on projects at Karolinska Institutet partly financed by investigator‐initiated grants from Janssen and Ferring, and also reports grants from Pfizer, Janssen, AbbVie, and Alfasigma in the context of a national safety monitoring programs. None of those studies have any relation to the present study. Karolinska Institutet also has received fees for Olén's lectures and participation on advisory boards from Janssen, Ferring, Takeda, and Pfizer regarding topics not related to the present study. Michael Eberhardson: Eberhardson is employed by AstraZeneca and has shares in AstraZeneca. Eberhardson has received honoraria for lectures and consultancy from AbbVie, Merck (MSD), Takeda, Ferring, Orion Pharma, Otsuka, Tillotts, ITH, Novartis, Pfizer, Oxion Biologics, Janssen, Bristol‐Myers Squibb, and Galapagos and received research funding from AbbVie and MSD.
Figures

Similar articles
-
Risk factors for anastomotic recurrence after primary ileocaecal resection in Crohn's disease.Eur J Gastroenterol Hepatol. 2018 Oct;30(10):1143-1147. doi: 10.1097/MEG.0000000000001206. Eur J Gastroenterol Hepatol. 2018. PMID: 30024490
-
Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: a randomised controlled, open-label, multicentre trial.Lancet Gastroenterol Hepatol. 2017 Nov;2(11):785-792. doi: 10.1016/S2468-1253(17)30248-0. Epub 2017 Aug 31. Lancet Gastroenterol Hepatol. 2017. PMID: 28838644 Clinical Trial.
-
Laparoscopic ileocaecal resection versus infliximab for terminal ileitis in Crohn's disease: retrospective long-term follow-up of the LIR!C trial.Lancet Gastroenterol Hepatol. 2020 Oct;5(10):900-907. doi: 10.1016/S2468-1253(20)30117-5. Epub 2020 Jun 30. Lancet Gastroenterol Hepatol. 2020. PMID: 32619413 Clinical Trial.
-
Preventing Recurrence of Crohn's Disease Post-Ileocaecal Surgery in Paediatric Patients: A Therapy Guide Based on Systematic Review of the Evidence.Paediatr Drugs. 2024 Nov;26(6):659-672. doi: 10.1007/s40272-024-00650-w. Epub 2024 Aug 31. Paediatr Drugs. 2024. PMID: 39215954
-
Pre-operative management is associated with low rate of post-operative morbidity in penetrating Crohn's disease.Aliment Pharmacol Ther. 2010 Aug;32(3):459-65. doi: 10.1111/j.1365-2036.2010.04369.x. Epub 2010 May 22. Aliment Pharmacol Ther. 2010. PMID: 20497144 Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
