Determinants of late metastases in renal cell carcinoma
- PMID: 40065697
- PMCID: PMC12229465
- DOI: 10.1093/jnci/djaf060
Determinants of late metastases in renal cell carcinoma
Abstract
Background: The mechanisms underlying metastatic latency in renal cell carcinoma (RCC) remain poorly understood.
Methods: This study evaluated 2 large independent cohorts for differences in tumor biology between patients who developed metastases early (≤1 year after nephrectomy) and those with late onset (>3 years).
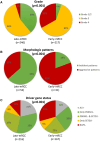
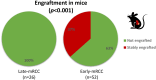
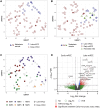
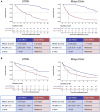
Results: In the discovery cohort (n = 161), late metastatic RCC was associated with clear cell histology (88.9% vs 78.7%), lower pathological stage (pT1-2; 40.3% vs 18.0%), and favorable histopathological features including low grade (40.0% vs 2.3%), less sarcomatoid (5.6% vs 21.8%), and reduced necrosis (37.7% vs 78.3%; all P < .02). Late metastatic RCC tumors exhibited increased angiogenesis (63.5% vs 19.4%) and reduced inflammation (78.8% vs 50.0%; all P < .02) profiles. Genomic driver analyses revealed comparable rates of PBRM1 and SETD2 loss in late and early metastatic RCC, while BAP1 loss was significantly less common in late metastatic RCC (7.5% vs 27.1%; P < .02). In multivariable models, BAP1/PBRM1/SETD2 status and tumor necrosis emerged as key discriminators of late metastatic RCCs. These findings were confirmed in the second cohort (n = 307). Late metastatic RCC was enriched for fatty acid oxidation and angiogenesis pathways, supporting a less aggressive phenotype. This was further evidenced by a lower engraftment rate in murine models (0% vs 36.5%; P < .001) and significantly longer overall survival from the time of metastasis (median survival doubled, P < .001). Interestingly, late metastatic RCC shared genomic and phenotypic features with RCC that metastasizes to the pancreas, suggesting a common underlying biology influencing both metastatic latency and pancreatic tropism.
Conclusions: Overall, these findings advocate for recognition of late metastatic RCC because of its distinct biology and improved prognosis.
Published by Oxford University Press 2025.
Conflict of interest statement
No relevant conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

