Multiple Pathways of Visual Adaptations for Water Column Usage in an Antarctic Adaptive Radiation
- PMID: 40065920
- PMCID: PMC11890982
- DOI: 10.1002/ece3.70867
Multiple Pathways of Visual Adaptations for Water Column Usage in an Antarctic Adaptive Radiation
Abstract
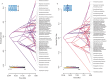
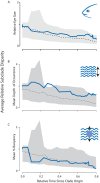
Evolutionary transitions in water column usage have played a major role in shaping ray-finned fish diversity. However, the extent to which vision-associated trait complexity and water column usage is coupled remains unclear. Here we investigated the relationship between depth niche, eye size, and the molecular basis of light detection across the Antarctic notothenioid adaptive radiation. Integrating a phylogenetic comparative framework with data on eye size and depth occupancy, we provide support for an acceleration in the rate of eye size diversification nearly 20 million years after the initial radiation. Our results further reveal that levels of eye size divergence are often highest between closely related taxa. We further analyzed opsin tuning site sequences and found changes representing repeated instances of independent tuning site changes across the notothenioid phylogeny that are generally not associated with habitat depth or species eye size. Collectively, our results strongly support that multiple evolutionary pathways underlie the diversification of visual adaptations in this iconic adaptive radiation.
Keywords: icefish; notothenioids; opsin; tuning site.
© 2025 The Author(s). Ecology and Evolution published by John Wiley & Sons Ltd. This article has been contributed to by U.S. Government employees and their work is in the public domain in the USA.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Abrams, P. A. 2014. “How Precautionary Is the Policy Governing the Ross Sea Antarctic Toothfish (Dissostichus Mawsoni) Fishery?” Antarctic Science 26, no. 1: 3–14.
-
- Alfaro, M. E. , Bolnick D. I., and Wainwright P. C.. 2005. “Evolutionary Consequences of Many‐To‐One Mapping of Jaw Morphology to Mechanics in Labrid Fishes.” American Naturalist 165, no. 6: E140–E154. - PubMed
-
- Balguerias, E. 2000. “Changes in Biomass of Eight Species of Finfish Around the South Orkney Islands (Subarea 48.2) From Three Bottom Trawl Surveys.” CCAMLR Science 7: 53–74.
-
- Benjamini, Y. , and Hochberg Y.. 1995. “Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing.” Journal of the Royal Statistical Society 57, no. 1: 289–300.
-
- Berner, D. 2011. “Size Correction in Biology: How Reliable Are Approaches Based on (Common) Principal Component Analysis?” Oecologia 166, no. 4: 961–971. - PubMed
LinkOut - more resources
Full Text Sources

