Artificial plasticenta: how polystyrene nanoplastics affect in-vitro cultured human trophoblast cells
- PMID: 40066256
- PMCID: PMC11891164
- DOI: 10.3389/fcell.2025.1539600
Artificial plasticenta: how polystyrene nanoplastics affect in-vitro cultured human trophoblast cells
Abstract
Background: In the human placenta, we have detected the MPs by Raman microspectroscopy analysis and, for the first time, with transmission electron microscopy. MPs fragments have been localized in different compartments of placental tissue, free in the cytoplasm and within organelles like lysosomes. Moreover, their presence has been correlated with ultrastructural alterations of some cell organelles, typical of metabolic stress, mainly dilated rough endoplasmic reticulum and numerous swollen electrodense mitochondria, as well as signs derived from involuting organelles. As a result, we have speculated that microplastics in the placenta could be responsible for pathological traits activation such as oxidative stress, apoptosis, and inflammation causing long-term effects on the health of the mother and child. To demonstrate the cytotoxicity of PS-NPs on the placenta and confirm the in vivo results, we performed in vitro experiments on a trophoblast human cell line, the HTR8/SVneo cells.
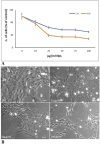
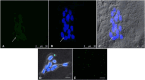
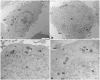
Materials and methods: HTR8/SVneo cells were treated, for 24 h and 48h, with increasing concentrations (10, 25, 50, 75, and 100 μg/mL) of 0.05 µm polystyrene (PS) and cellular viability was evaluated by Counting Kit-8. Fluorescent PS-NPs examined under fluorescence/confocal microscopy were used to investigate the internalization of plastics in the placenta cells. Transmission electron microscopy was used to evaluate possible PS-NPs-dependent ultrastructural alterations of cells and organelles.
Results: Our study shows that starting from 24 h exposure, PS-NPs treatment, at 50 μg/mL dose, has a cytotoxic effect on placental cells, causing the death of 40% of cells and affecting the morphology of the surviving cells. In addition, PS-NPs alter the ultrastructure of some organelles in the surviving cells, like those we have already described in vivo. We found that NPs enter the cells, affecting the endoplasmic reticulum and mitochondria morphology, accumulating as aggregates within lysosome-like organelles. Interestingly these aggregates become larger as the concentration of NPs increases. We speculated that the accumulation of NPs inside lysosome-like organelles could result from a prolonged and impossible attempt by the cell to remove and destroy PS. This would lead to ER and mitochondrial stress, impairing mitochondria/ER functions and oxidative stress, thus activating the apoptotic pathway and suggesting that PS-NPs could act as a cell stressor, leading to the death of cells. In support of our hypothesis, we also found NPs associated with morphological signs of cellular regression and degeneration, such as the presence of a highly vacuolized cytoplasm, dilatation, and vesiculation of ER, associated with the uncoupling/loss of associated mitochondria, cytoplasmic fragments, and free organelles deriving from cellular lysis.
Conclusion: Based on electron microscopy and immunofluorescence analysis and in vitro study, we demonstrate the cytotoxicity of PS-NPs in trophoblast cells together with ultrastructural alterations associated with cellular regression and degeneration typical of metabolic stress. An abnormal amount of NPs in the cells might determine a persistent cellular alarm CDR (cell danger response), the evolutionarily conserved metabolic response that protects the cells and hosts from harm triggered by chemical (as in the case of NPs/MPs), physical, or biological agents that exceed the cellular capacity for homeostasis. This in vitro study could further help to demonstrate that the inevitable exposure of MPs/NPs in the environment, which characterizes the modern world, might be partially responsible for the epidemic of non-transmissible disease.
Keywords: altered fetal programming; confocal and transmission electron microscopy; placenta; plastics; polystyrene (PS) nanoplastics; ultrastructure.
Copyright © 2025 Ragusa, Cristiano, Di Vinci, Familiari, Nottola, Macchiarelli, Svelato, De Luca, Rinaldo, Neri and Facchinetti.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures






References
-
- Ahamed M., Akhtar M. J. (2024). Cytotoxic effect of polystyrene nanoplastics in human umbilical vein endothelial cells (HUVECs) and normal rat kidney cells (NRK52E). J. King Saud Univ. – Sci. 36, 103506. 10.1016/j.jksus.2024.103505 - DOI
-
- Alimba C. G., Faggio C., Sivanesan S., Ogunkanmi A. L., Krishnamurthi K. (2021). Micro(nano)-plastics in the environment and risk of carcinogenesis: insight into possible mechanisms. J. Hazard. Mat. 416, 126143. 10.1016/j.jhazmat.2021.126143 - DOI
-
- Amato-Lourenço L. F., dos Santos Galvão L., Letti A., de Weger L. A., Hiemstra P. S., Vijver M. G., et al. (2020). An emerging class of air pollutants: potential effects of microplastics to respiratory human health? Sci.Total. Environ. 749, 141676. 10.1016/j.scitotenv.2020.141676 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

