Intestinal current measurement detects age-dependent differences in CFTR function in rectal epithelium
- PMID: 40066329
- PMCID: PMC11891205
- DOI: 10.3389/fphar.2025.1537095
Intestinal current measurement detects age-dependent differences in CFTR function in rectal epithelium
Abstract
Objective: Intestinal current measurement (ICM) provides a sensitive bioassay for assessment of cystic fibrosis transmembrane conductance regulator (CFTR) function in rectal biopsies ex vivo and is used as a diagnostic tool for cystic fibrosis (CF). Furthermore, ICM was shown to be sensitive to detect pharmacological rescue of CFTR function by CFTR modulators in people with CF carrying responsive CFTR mutations. Results from clinical trials of CFTR modulators across age groups indicate that CFTR function in the sweat duct may be age-dependent with children reaching higher levels than adults. However, little is known about age dependency of CFTR function in the intestinal epithelium.
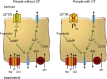
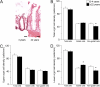
Methods: We investigated CFTR-mediated chloride secretion in rectal biopsies from 258 people without CF and 72 people with pancreatic-insufficient CF from 1 month to 68 years of age. Change in transepithelial short-circuit current in response to cyclic adenosine monophosphate (cAMP)-mediated (100 μM IBMX, 1 µM forskolin, basolateral) and cholinergic (100 μM carbachol, basolateral) stimulation was assessed as a readout for CFTR function using perfused micro-Ussing chambers. Furthermore, quantitative real-time PCR of CFTR and morphometric analysis of epithelial cells lining the crypts and surface of the rectal mucosa were performed to assess regulation at the levels of gene expression and epithelial cell densities.
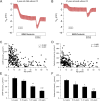
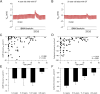
Results: We found that CFTR-mediated chloride secretion across rectal tissues, as determined from cAMP-mediated as well as cholinergic chloride-secretory responses was highest during infancy and early childhood and declined with age in people without CF (both P < 0.001). Although, there was no difference in cAMP-mediated currents in people with CF, potassium-secretory responses induced by cholinergic stimulation were also reduced with increasing age. Transcript analyses showed that CFTR mRNA expression was slightly increased with increasing age in people without CF (P < 0.05). Morphometric analyses demonstrated that CFTR expressing colonocytes at the crypt base were decreased with age (P < 0.05). A secondary analysis of the ICM data of our previous studies on the effects of lumacaftor/ivacaftor on CFTR function in F508del -homozygous people with CF aged 12 years and older and 2-11 year old children showed correlations of the change in cAMP-mediated and cholinergic chloride secretory response with the age of people with CF (P < 0.01 and P < 0.05, respectively).
Conclusion: These results demonstrate that CFTR function in the rectal epithelium is reduced with increasing age and indicate that this change is likely due to a decline in the number of secretory colonocytes at the crypt base. These findings suggest that differences in CFTR expressing cells may explain increased functional responses to CFTR modulator therapies in children compared to adult people with CF.
Keywords: CFTR; CFTR modulator therapy; age-dependency; intestinal current measurement; rectal epithelium; secretory diarrhea.
Copyright © 2025 Graeber, Sommerburg, Yu, Berges, Hirtz, Scheuermann, Berger, Duerr and Mall.
Conflict of interest statement
SYG reports grants from Mukoviszidose e.V. (German CF Foundation) and Vertex Pharmaceuticals Incorporated outside the submitted work, with payments made to institution; personal fees for advisory board participation from Chiesi GmbH and Vertex Pharmaceuticals Incorporated; lecture honoraria and honoraria for a CME module from Vertex Pharmaceuticals Incorporated. OS reports grants from Vertex Pharmaceuticals Incorporated outside the submitted work, with payments made to institution; lecture honoraria from Teva GmbH and Vertex Pharmaceuticals Incorporated. YY reports grants from Mukoviszidose e.V. (German CF Foundation). MAM reports grants from the German Research Foundation (DFG), the German Federal Ministry of Education and Research (BMBF), and an independent medical grant from Vertex Pharmaceuticals, with payments made to the institution; personal fees for advisory board participation or consulting from Boehringer Ingelheim, Enterprise Therapeutics, Kither Biotech, Splisense, and Vertex Pharmaceuticals; lecture honoraria from Vertex Pharmaceuticals; and travel support from Boehringer Ingelheim and Vertex Pharmaceuticals; and is unpaid Associate Editor of the European Respiratory Journal and Fellow of the European Respiratory Society (FERS). The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Berges J., Graeber S. Y., Hammerling S., Yu Y., Krumpelmann A., Stahl M., et al. (2023). Effects of lumacaftor-ivacaftor therapy on cystic fibrosis transmembrane conductance regulator function in F508del homozygous patients with cystic fibrosis aged 2-11 years. Front. Pharmacol. 14, 1188051. 10.3389/fphar.2023.1188051 - DOI - PMC - PubMed
-
- Birch R. J., Peckham D., Wood H. M., Quirke P., Konstant-Hambling R., Brownlee K., et al. (2023). The risk of colorectal cancer in individuals with mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) gene: an English population-based study. J. Cyst. Fibros. 22 (3), 499–504. 10.1016/j.jcf.2022.10.001 - DOI - PubMed
LinkOut - more resources
Full Text Sources

