Update on tocilizumab in rheumatoid arthritis: a narrative review
- PMID: 40066438
- PMCID: PMC11891176
- DOI: 10.3389/fimmu.2025.1470488
Update on tocilizumab in rheumatoid arthritis: a narrative review
Abstract
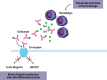
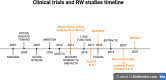
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterized by joint pain, swelling, and stiffness, affecting approximately 1% of the adult population. Tocilizumab (TCZ), a monoclonal antibody targeting the IL-6 receptor, has emerged as an effective treatment for RA. This narrative review provides an update on TCZ's efficacy and safety based on data from randomized controlled trials (RCTs) and real-world evidence (RWE). TCZ, available in subcutaneous (SC) and intravenous (IV) formulations, has shown significant benefits in RA management. Key clinical trials, including SAMURAI, OPTION, RADIATE, and TOWARD, have demonstrated TCZ's efficacy as monotherapy and in combination with conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), particularly in patients with inadequate responses to methotrexate or TNF inhibitors. Long-term studies, such as STREAM, have highlighted TCZ's sustained efficacy and favorable safety profile over 5 years. The impact of TCZ on cardiovascular health, lipid profiles, and the risk of infections has been a focal point, with findings suggesting no significant increase in cardiovascular disease risk compared to other RA therapies. RWE further highlights the effectiveness of TCZ, identifying predictors of response, such as age, and emphasizes its suitability for biologic-naïve and overweight patients. Special considerations include TCZ use in RA-associated interstitial lung disease and amyloidosis. Overall, TCZ remains a pivotal option in RA treatment, with a well-established safety and efficacy profile supported by extensive clinical and real-world data.
Keywords: IL-6 receptor inhibitor; efficacy and safety; randomized controlled trials (RCTs); real-world evidence (RWE); rheumatoid arthritis (RA); tocilizumab (TCZ).
Copyright © 2025 Parisi, Ditto, Ghellere, Panaro, Piccione, Borrelli and Fusaro.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Strand V, Burmester GR, Ogale S, Devenport J, John A, Emery P. Improvements in health-related quality of life after treatment with tocilizumab in patients with rheumatoid arthritis refractory to tumour necrosis factor inhibitors: results from the 24-week randomized controlled RADIATE study. Rheumatol (Oxford). (2012) 51:1860–9. doi: 10.1093/rheumatology/kes131 - DOI - PMC - PubMed
-
- Cacciapaglia F, Spinelli FR, Bartoloni E, Bugatti S, Erre GL, Fornaro M, et al. . Clinical features of diabetes mellitus on rheumatoid arthritis: data from the Cardiovascular Obesity and Rheumatic DISease (CORDIS) study group. J Clin Med. (2023) 12:2148. doi: 10.3390/jcm12062148 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical