In-depth analysis of the safety of CAR-T cell therapy for solid tumors
- PMID: 40066440
- PMCID: PMC11891211
- DOI: 10.3389/fimmu.2025.1548979
In-depth analysis of the safety of CAR-T cell therapy for solid tumors
Abstract
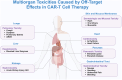
In recent years, the rapid progress in oncology, immunology, and molecular biology has dramatically advanced cancer immunotherapy, particularly CAR-T cell therapy. This innovative approach involves engineering a patient's T cells to express receptors that specifically target tumor antigens, enhancing their ability to identify and eliminate cancer cells. However, the effectiveness of CAR-T therapy in solid tumors is often hampered by the challenging tumor microenvironment (TME). The complex TME includes dense stroma that obstructs T cell infiltration, abnormal blood vessel structures leading to hypoxia, and an acidic pH, all of which hinder CAR-T cell function. Additionally, the presence of immunosuppressive factors in the TME reduces the efficacy of CAR-T cells, making successful targeting of tumors more difficult. The safety of CAR-T therapy has gained interest, especially CAR-T therapy has shown considerable effectiveness in various cancers, with notable results in multiple myeloma and hepatocellular carcinoma, among others. Nonetheless, CAR-T cell therapy is associated with several adverse reactions primarily driven by heightened levels of proinflammatory cytokines. These reactions include cytokine release syndrome (CRS), neurotoxicity (CANS), and organ toxicity, often leading to serious complications. CRS, characterized by systemic inflammation due to cytokine release, can escalate to severe organ dysfunction. It typically occurs within the first week post-infusion, correlating with CAR-T cell expansion and often presents with fever and hypotension. Meanwhile, CANS encompasses neurological issues ranging from mild symptoms to severe seizures, possibly exacerbated by CRS. Organ toxicity can also arise from CAR-T therapy, with potential damage affecting the gastrointestinal tract, kidneys, liver, and lungs, often tied to shared antigens found in both tumor and healthy tissues. Moreover, long-term effects like cytokine-associated hematotoxicity (CAHT) and secondary malignancies represent significant concerns that could affect the patient's quality of life post-treatment. The long-term adverse effects and challenges in treating solid tumors underscore the need for ongoing research. Strategies to improve CAR-T cell efficacy, minimize adverse reactions, and enhance patient safety are critical. Future explorations could include designing CAR-T cells to better navigate the TME, identifying specific target antigen profiles to minimize off-target damage, and developing adjunct therapies to mitigate cytokine-related toxicity. Continued monitoring for long-term effects will also be paramount in improving patient outcomes and maintaining their quality of life. Overall, while CAR-T therapy holds great promise, it must be administered with careful consideration of potential side effects and rigorous management strategies to ensure patient safety and treatment efficacy.
Keywords: CAR-T cells; adverse reactions; solid tumors; tumor immunity; tumor therapy.
Copyright © 2025 Dong, Wu, Jin, Zheng, Su, Shao, Bei and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

