Transthyretin amyloid cardiomyopathy in aortic stenosis patients scheduled for transcatheter aortic valve implantation
- PMID: 40066648
- PMCID: PMC12287841
- DOI: 10.1002/ehf2.15258
Transthyretin amyloid cardiomyopathy in aortic stenosis patients scheduled for transcatheter aortic valve implantation
Abstract
Aims: The prevalences of aortic stenosis (AS) and transthyretin amyloid cardiomyopathy (ATTR-CM) increase with age. Identification of occult ATTR-CM in patients with AS can help explain out-of-proportion myocardial dysfunction, aid in prognostication and prompt initiation of disease-modifying treatment. Studies have suggested that many patients referred for transcatheter aortic valve implantation (TAVI) have concomitant ATTR-CM, but some have included unverified ATTR-CM in patients with ambiguous scintigrams. We aimed to assess the co-occurrence of ATTR-CM in patients scheduled for TAVI.
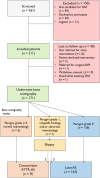
Methods: In patients accepted for TAVI for severe AS, we used bone scintigraphy to screen for ATTR-CM. We defined ATTR-CM as a cardiac tracer uptake ≥ Perugini Grade 2 in the absence of abnormal monoclonal protein or abnormal free light chain ratio. We offered bioptic verification to patients with Grade 1 or ambiguous DPD uptake.
Results: We included 171 consecutive patients aged 79 ± 7 years, 57% were male. Six patients (3.5%) had cardiac bone tracer uptake ≥ Perugini Grade 2 and no abnormal monoclonal protein/free light chains. Endomyocardial biopsies confirmed the diagnosis in two additional patients (1.2%), whereas five patients with low-grade uptake did not have ATTR-CM. In total, 8/171 patients (4.7%) were diagnosed with concomitant AS and ATTR-CM. Most of the patients with concomitant ATTR-CM had low-flow low-gradient (LFLG) AS, and 25% had a history of carpal tunnel syndrome.
Conclusions: We found concomitant AS and ATTR-CM in 5% of our TAVI patients. Carpal tunnel syndrome and LFLG AS suggest concomitant ATTR.
Keywords: ATTR‐CM; TAVI; aortic stenosis; bone scintigraphy; cardiac amyloidosis; endomyocardial biopsy.
© 2025 The Author(s). ESC Heart Failure published by John Wiley & Sons Ltd on behalf of European Society of Cardiology.
Conflict of interest statement
Dr. Gullestad has received lecture fees from AstraZeneca, Boehringer Ingelheim and Novartis and has sat on advisory boards for AstraZeneca and Boehringer Ingelheim. Dr. Broch has received lecture fees and consulting fees from Pfizer and has sat on advisory boards for AstraZeneca, Pharmacosmos, Boehringer Ingelheim and Pfizer. Dr. Gude has received grants and honoraria for lectures from Pfizer and has sat on advisory boards for Pfizer. Dr. Hodt has received honoraria for lectures from Pfizer. The remaining authors have no conflict of interest to disclose.
Figures
References
-
- Osnabrugge RL, Arnold SV, Reynolds MR, Magnuson EA, Wang K, Gaudiani VA, et al. Health status after transcatheter aortic valve replacement in patients at extreme surgical risk: results from the CoreValve U.S. trial. JACC Cardiovasc Interv 2015;8:315‐323. doi: 10.1016/j.jcin.2014.08.016 - DOI - PMC - PubMed
-
- Gilstrap LG, Dominici F, Wang Y, El‐Sady MS, Singh A, Di Carli MF, et al. Epidemiology of cardiac amyloidosis‐associated heart failure hospitalizations among fee‐for‐service Medicare beneficiaries in the United States. Circ Heart Fail 2019;12:e005407. doi: 10.1161/CIRCHEARTFAILURE.118.005407 - DOI - PMC - PubMed
MeSH terms
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials