Adjuvant Aspirin Treatment in PIK3CA-Mutated Colon Cancer Patients: The SAKK 41/13 Prospective Randomized Placebo-Controlled Double-Blind Trial
- PMID: 40067142
- PMCID: PMC7617601
- DOI: 10.1158/1078-0432.CCR-24-4048
Adjuvant Aspirin Treatment in PIK3CA-Mutated Colon Cancer Patients: The SAKK 41/13 Prospective Randomized Placebo-Controlled Double-Blind Trial
Abstract
Purpose: We assessed the benefit of adjuvant aspirin in patients with resected phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit alpha (PIK3CA)-mutated colon cancer.
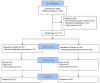
Patients and methods: This was a phase III, prospective, randomized, placebo-controlled, double-blind, multicenter, and multinational trial. Patients with resected colon cancer stage II and III harboring an activating PIK3CA mutation were included. Because of financial constraints, the trial was prematurely closed. Randomization was 2:1 to aspirin 100 mg versus placebo daily for 3 years. The primary endpoint was disease-free survival (DFS). Secondary endpoints included the time to disease recurrence (TTR), overall survival, and adverse events.
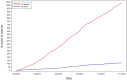
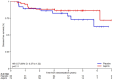
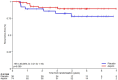
Results: Overall, 1,040 patients were screened for PIK3CA mutations, with 112 randomized to aspirin (N = 74) and placebo (N = 38). The median age was 66 years, and 42.9% were female. After a median follow-up of 4 years, 19 DFS events occurred, including 10 in the aspirin and nine in the placebo arm. The HR for DFS was 0.57 [90% confidence interval (CI), 0.27-1.22], in favor of aspirin (P = 0.11). DFS rates at 5 years were 86.5% (90% CI, 77.7%-92.0%) in the aspirin and 72.9% (90% CI, 55.7%-84.3%) in the placebo arm. The HR for TTR was 0.49 (90% CI, 0.21-1.19, P = 0.089) in favor of aspirin. No patient experienced aspirin-related serious adverse events.
Conclusions: The SAKK 41/13 is the first randomized trial to provide clinical evidence of a protective effect of adjuvant aspirin in patients with resected PIK3CA-mutant colon cancer, with clinically relevant DFS and TTR improvements. Although results were not statistically significant because of premature study closure, adjuvant aspirin warrants individual consideration in patients with resected PIK3CA-mutant colon cancer stage II and III. See related commentary by Drew et al., p. 3107.
©2025 American Association for Cancer Research.
Conflict of interest statement
Figures
References
-
- Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74(1):12–49. - PubMed
-
- Argilés G, Tabernero J, Labianca R, Hochhauser D, Salazar R, Iveson T, et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(10):1291–1305. - PubMed
-
- Souglakos J, Boukovinas I, Kakolyris S, Xynogalos S, Ziras N, Athanasiadis A, et al. Three- versus six-month adjuvant FOLFOX or CAPOX for high-risk stage II and stage III colon cancer patients: the efficacy results of Hellenic Oncology Research Group (HORG) participation to the International Duration Evaluation of Adjuvant Chemotherapy (IDEA) project. Ann Oncol. 2019;30(8):1304–1310. - PubMed
-
- Sobrero AF, Puccini A, Shi Q, Grothey A, Andrè T, Shields AF, et al. A new prognostic and predictive tool for shared decision making in stage III colon cancer. Eur J Cancer. 2020;138:182–188. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

