The deubiquitinase USP45 inhibits autophagy through actin regulation by Coronin 1B
- PMID: 40067150
- PMCID: PMC11895698
- DOI: 10.1083/jcb.202407014
The deubiquitinase USP45 inhibits autophagy through actin regulation by Coronin 1B
Erratum in
-
Correction: The deubiquitinase USP45 inhibits autophagy through actin regulation by Coronin 1B.J Cell Biol. 2025 May 5;224(5):e20240701403262025c. doi: 10.1083/jcb.20240701403262025c. Epub 2025 Apr 4. J Cell Biol. 2025. PMID: 40184486 Free PMC article. No abstract available.
Abstract
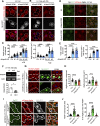
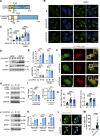
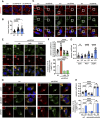
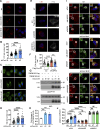
The autophagy-lysosomal system comprises a highly dynamic and interconnected vesicular network that plays a central role in maintaining proteostasis and cellular homeostasis. In this study, we uncovered the deubiquitinating enzyme (DUB), dUsp45/USP45, as a key player in regulating autophagy and lysosomal activity in Drosophila and mammalian cells. Loss of dUsp45/USP45 results in autophagy activation and increased levels of V-ATPase to lysosomes, thus enhancing lysosomal acidification and function. Furthermore, we identified the actin-binding protein Coronin 1B (Coro1B) as a substrate of USP45. USP45 interacts with and deubiquitinates Coro1B, thereby stabilizing Coro1B levels. Notably, the ablation of USP45 or Coro1B promotes the formation of F-actin patches and the translocation of V-ATPase to lysosomes in an N-WASP-dependent manner. Additionally, we observed positive effects of dUsp45 depletion on extending lifespan and ameliorating polyglutamine (polyQ)-induced toxicity in Drosophila. Our findings highlight the important role of dUsp45/USP45 in regulating lysosomal function by modulating actin structures through Coro1B.
© 2025 Lin et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures








References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

