Clinical Outcomes of Hypertonic Saline vs Mannitol Treatment Among Children With Traumatic Brain Injury
- PMID: 40067302
- PMCID: PMC11897838
- DOI: 10.1001/jamanetworkopen.2025.0438
Clinical Outcomes of Hypertonic Saline vs Mannitol Treatment Among Children With Traumatic Brain Injury
Erratum in
-
Errors in Table.JAMA Netw Open. 2025 May 1;8(5):e2515833. doi: 10.1001/jamanetworkopen.2025.15833. JAMA Netw Open. 2025. PMID: 40358953 Free PMC article. No abstract available.
Abstract
Importance: The use of hypertonic saline (HTS) vs mannitol in the control of elevated intracranial pressure (ICP) secondary to neurotrauma is debated.
Objective: To compare mortality and functional outcomes of treatment with 3% HTS vs 20% mannitol among children with moderate to severe traumatic brain injury (TBI) at risk of elevated ICP.
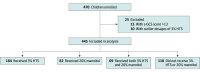
Design, setting, and participants: This prospective, multicenter cohort study was conducted between June 1, 2018, and December 31, 2022, at 28 participating pediatric intensive care units in the Pediatric Acute and Critical Care Medicine in Asia Network (PACCMAN) and the Red Colaborativa Pediátrica de Latinoamérica (LARed) in Asia, Latin America, and Europe. The study included children (aged <18 years) with moderate to severe TBI (Glasgow Coma Scale [GCS] score ≤13).
Exposure: Treatment with 3% HTS compared with 20% mannitol.
Main outcomes and measures: Multiple log-binomial regression analysis was performed for mortality, and multiple linear regression analysis was performed for discharge Pediatric Cerebral Performance Category (PCPC) scores and 3-month Glasgow Outcome Scale-Extended Pediatric Version (GOS-E-Peds) scores. Inverse probability of treatment weighting was also performed using the propensity score method to control for baseline imbalance between groups.
Results: This study included 445 children with a median age of 5.0 (IQR, 2.0-11.0) years. More than half of the patients (279 [62.7%]) were boys, and 344 (77.3%) had severe TBI. Overall, 184 children (41.3%) received 3% HTS, 82 (18.4%) received 20% mannitol, 69 (15.5%) received both agents, and 110 (24.7%) received neither agent. The mortality rate was 7.1% (13 of 184 patients) in the HTS group and 11.0% (9 of 82 patients) in the mannitol group (P = .34). After adjusting for age, sex, presence of child abuse, time between injury and hospital arrival, lowest GCS score in the first 24 hours, and presence of extradural hemorrhage, no between-group differences in mortality, hospital discharge PCPC scores, or 3-month GOS-E-Peds scores were observed.
Conclusions and relevance: In this cohort study of children with moderate to severe TBI, the use of HTS was not associated with increased survival or improved functional outcomes compared with mannitol. Future large multicenter randomized clinical trials are required to validate these findings.
Conflict of interest statement
Figures
References
-
- James SL, Theadom A, Ellenbogen RG, et al. ; GBD 2016 Traumatic Brain Injury and Spinal Cord Injury Collaborators . Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18(1):56-87. doi: 10.1016/S1474-4422(18)30415-0 - DOI - PMC - PubMed
-
- Kochanek PM, Tasker RC, Carney N, et al. Guidelines for the management of pediatric severe traumatic brain injury, third edition: update of the Brain Trauma Foundation guidelines, executive summary. Pediatr Crit Care Med. 2019;20(3):280-289. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical