Transcriptomic profiling after B cell depletion reveals central and peripheral immune cell changes in multiple sclerosis
- PMID: 40067358
- PMCID: PMC12126227
- DOI: 10.1172/JCI182790
Transcriptomic profiling after B cell depletion reveals central and peripheral immune cell changes in multiple sclerosis
Abstract
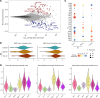
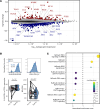
Multiple sclerosis (MS) is a complex, genetically mediated autoimmune disease of the CNS, in which anti-CD20-mediated B cell depletion is remarkably effective in the treatment of early disease. Although previous studies investigated the effect of B cell depletion on select immune cell subsets using flow cytometry-based methods, the therapeutic effect on the patient's immune landscape is unknown. In this study, we explored how B cell-depleting therapies modulate the immune landscape using single-cell RNA-Seq. We demonstrate that B cell depletion led to cell-type-specific changes in the abundance and function of cerebrospinal fluid (CSF) macrophages and peripheral blood monocytes. Specifically, a CSF-specific macrophage population with an antiinflammatory transcriptomic signature and peripheral CD16+ monocytes increased in frequency after B cell depletion. This was accompanied by increases in TNF-α mRNA and protein levels in monocytes following B cell depletion, consistent with the finding that anti-TNF-α treatment exacerbated autoimmune activity in MS. In parallel, B cell depletion induced changes in peripheral CD4+ T cell populations, including increases in the frequency of TIGIT+ Tregs and marked decreases in the frequency of myelin peptide-loaded, tetramer-binding CD4+ T cells. Collectively, this study provides an exhaustive transcriptomic map of immunological changes, revealing different cell-type-specific reprogramming as a result of B cell depletion treatment of MS.
Keywords: Autoimmunity; Immunology; Multiple sclerosis.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

