Proteostasis and metabolic dysfunction characterize a subset of storage-induced senescent erythrocytes targeted for posttransfusion clearance
- PMID: 40067362
- PMCID: PMC12043093
- DOI: 10.1172/JCI183099
Proteostasis and metabolic dysfunction characterize a subset of storage-induced senescent erythrocytes targeted for posttransfusion clearance
Abstract
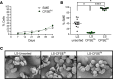
Although refrigerated storage slows the metabolism of volunteer donor RBCs, which is essential in transfusion medicine, cellular aging still occurs throughout this in vitro process. Storage-induced microerythrocytes (SMEs) are morphologically altered senescent RBCs that accumulate during storage and are cleared from circulation following transfusion. However, the molecular and cellular alterations that trigger clearance of this RBC subset remain to be identified. Using a staining protocol that sorts long-stored SMEs (i.e., CFSEhi) and morphologically normal RBCs (CFSElo), these in vitro aged cells were characterized. Metabolomics analysis identified depletion of energy, lipid-repair, and antioxidant metabolites in CFSEhi RBCs. By redox proteomics, irreversible protein oxidation primarily affected CFSEhi RBCs. By proteomics, 96 proteins, mostly in the proteostasis family, had relocated to CFSEhi RBC membranes. CFSEhi RBCs exhibited decreased proteasome activity and deformability; increased phosphatidylserine exposure, osmotic fragility, and endothelial cell adherence; and were cleared from the circulation during human spleen perfusion ex vivo. Conversely, molecular, cellular, and circulatory properties of long-stored CFSElo RBCs resembled those of short-stored RBCs. CFSEhi RBCs are morphologically and metabolically altered, have irreversibly oxidized and membrane-relocated proteins, and exhibit decreased proteasome activity. In vitro aging during storage selectively alters metabolism and proteostasis in these storage-induced senescent RBCs targeted for clearance.
Keywords: Cell biology; Cellular senescence; Hematology; Proteomics; Ubiquitin-proteosome system.
Figures





Update of
-
Proteostasis and metabolic dysfunction in a distinct subset of storage-induced senescent erythrocytes targeted for clearance.bioRxiv [Preprint]. 2024 Sep 12:2024.09.11.612195. doi: 10.1101/2024.09.11.612195. bioRxiv. 2024. Update in: J Clin Invest. 2025 Mar 11;135(9):e183099. doi: 10.1172/JCI183099. PMID: 39314353 Free PMC article. Updated. Preprint.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

