Effects of tucidinostat in adult T-cell leukemia/lymphoma in clinical practice
- PMID: 40067419
- PMCID: PMC12202688
- DOI: 10.1007/s12185-025-03963-9
Effects of tucidinostat in adult T-cell leukemia/lymphoma in clinical practice
Abstract
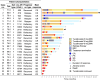
Adult T-cell leukemia/lymphoma (ATL) is a peripheral T-cell malignancy with a poor prognosis. We conducted a retrospective study across six institutions in Miyazaki Prefecture, Japan, to assess the efficacy of tucidinostat in patients with relapsed/refractory ATL who had not undergone transplantation. Between October 2021 and July 2023, 24 patients aged 41 to 88 years (median, 73.4 years) who had undergone prior therapies, including intensive chemotherapy (79.2%) and mogamulizumab immunotherapy (79.2%), received tucidinostat. Objective response rate (ORR), disease control rate (DCR), progression-free survival (PFS), and overall survival (OS) were evaluated as key outcomes. ORR and DCR reached 54.2% and 91.7%, respectively. The median PFS was 3.95 months, and OS was 8.04 months, which were not inferior to the results of a phase IIb study. The influential factors for PFS were age ≥ 75 years and high soluble IL-2 receptor (sIL-2R) levels above 5000 U/mL at the start of treatment. Favorable patients without these factors achieved a PFS of 11.4 months. Treatment-related adverse events were mainly hematologic but were managed over the course of treatment. Our findings indicate that tucidinostat provides survival benefits in patients with relapsed/refractory ATL in clinical practice and highlight key clinical factors for better outcomes.
Keywords: Adult T-cell leukemia/lymphoma; HDAC inhibitor; Tucidinostat.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: K. Shimoda received consulting fees from Novartis Pharma, Takeda Pharmaceutical, and Bristol-Myers, all outside the submitted work, and received research grants from Perseus Proteomics, Pharma Essentia Japan KK, AbbVie GK, Astellas Pharma, MSD, Chugai Pharmaceutical, Kyowa Kirin, Pfizer, Novartis Pharma, Otsuka Pharmaceutical, Meiji Seika Pharma, and Asahi Kasei Medical, all outside the submitted work.
Figures

References
-
- Uchiyama T, Yodoi J, Sagawa K, Takatsuki K, Uchino H. Adult T-cell leukemia: clinical and hematologic features of 16 cases. Blood. 1977;50(3):481–92. - PubMed
-
- Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984–87). Br J Haematol. 1991;79(3):428–37. - PubMed
-
- Shimoyama M. Chemotherapy of ATL. K Takatsuki (Ed), Adult T-Cell Leukemia, Oxford University Press, Oxford. 1994:221–227.
-
- Tsukasaki K, Utsunomiya A, Fukuda H, Shibata T, Fukushima T, Takatsuka Y, et al. VCAP-AMP-VECP compared with biweekly CHOP for adult T-cell leukemia-lymphoma: Japan Clinical Oncology Group Study JCOG9801. J Clin Oncol. 2007;25(34):5458–64. 10.1200/JCO.2007.11.9958. - PubMed
-
- Sekine M, Kameda T, Shide K, Maeda K, Toyama T, Kawano N, et al. Higher average chemotherapy dose intensity improves prognosis in patients with aggressive adult T-cell leukemia/lymphoma. Eur J Haematol. 2021;106(3):398–407. 10.1111/ejh.13565. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

