Verdiperstat in Amyotrophic Lateral Sclerosis: Results From the Randomized HEALEY ALS Platform Trial
- PMID: 40067754
- PMCID: PMC11833655
- DOI: 10.1001/jamaneurol.2024.5249
Verdiperstat in Amyotrophic Lateral Sclerosis: Results From the Randomized HEALEY ALS Platform Trial
Abstract
Importance: Myeloperoxidase is one of the most abundant peroxidase enzymes in activated myeloid cells. Myeloperoxidase inhibitors may have a clinical benefit in amyotrophic lateral sclerosis (ALS) by slowing neurodegeneration via reduced neuroinflammation and oxidative stress.
Objective: To determine the safety, tolerability, and efficacy of verdiperstat, a selective myeloperoxidase inhibitor, in ALS.
Design settings and participants: Verdiperstat was tested as a regimen of the HEALEY ALS Platform Trial, a multicenter, double-blind, perpetual platform design, randomized clinical trial, with sharing of trial infrastructure and placebo data across multiple regimens. The study was conducted at 54 ALS referral centers across the US from July 2020 to April 2022. Adult participants with a diagnosis of clinically possible, probable, laboratory-supported probable, or definite ALS defined by the revised El Escorial criteria were randomized to verdiperstat or regimen-specific placebo. An additional group of participants concurrently randomized to placebo from other regimens was included in the analyses.
Interventions: Eligible participants were randomized in a 3:1 ratio to receive oral verdiperstat, 600 mg, twice daily or matching placebo for a planned placebo-controlled duration of 24 weeks.
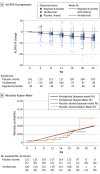
Main outcomes and measures: The primary efficacy outcome was change from baseline through week 24 in disease severity, as measured by a joint model of ALS Functional Rating Scale-Revised and survival, with the treatment effect quantified by the disease rate ratio (DRR), with DRR less than 1 indicating a slowing in disease progression of verdiperstat relative to placebo.
Results: A total of 167 participants (mean [SD] age, 58.5 [11.4] years; 59 [35.3%] female; 108 [64.6%] male) were randomized to either verdiperstat (126 [75.4%]) or to placebo (41 [25.6%]). Among the participants randomized to the verdiperstat regimen, 130 (78%) completed the trial. The estimated DRR was 0.98 (95% credible interval, 0.77-1.24; posterior probability = 0.57 for slowing of disease progression [DRR <1]). Verdiperstat was estimated to slow progression by 2% vs placebo (95% credible interval, -23% to 24%; posterior probability 0.57). Verdiperstat was overall safe and well tolerated. Common adverse events in the verdiperstat group were nausea, insomnia, and elevated thyrotropin levels.
Conclusions and relevance: Results demonstrate that treatment with verdiperstat was unlikely to alter disease progression in ALS.
Trial registration: Clinical Trial Identifiers: NCT04297683 and NCT04436510.
Copyright 2025 Writing Committee for the HEALEY ALS Platform Trial. JAMA Neurology.
Conflict of interest statement
Conflict of Interest Disclosures: Dr Andrews reported receiving data safety monitoring board fees from AL-S Pharma, Biogen, Cytokinetics, Amylyx, Quralis, Sanofi; grants from Amylyx, Biogen, Cytokinetics, Platform Trial [Ra/Biohaven/Clene/Prilenia/Calico], and the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) outside the submitted work. Dr Paganoni reported receiving grants from AMG Charitable Foundation, Tackle ALS, The ALS Association, ALS Finding a Cure, MDA, ALS ONE, Arthur Blank Family Foundation, I AM ALS, Tambourine, UCB, Biohaven, Clene, Amylyx, Eledon, Alector, Seelos, Prilenia, Calico, Denali, NIH, Centers for Disease Control and Prevention (CDC), Department of Defense; personal fees from Arrowhead, Biogen, J&J, Merck, Cytokinetics; consulting agreement from BMS, Clene, Prilenia, Eikonizo, Sola, PharmAust, Amylyx, Revalesio; speaker fees from PeerView, Medscape, and i3Health outside the submitted work. Dr Macklin reported receiving grants from Biohaven, AI Therapeutics, Alector, Biohaven, Calico Therapeutics, Denali Therapeutics, ITB-Med, Janssen, Lilly, Mitsubishi Tanabe Pharma America, Neurizon, Prilenia Therapeutics, Revalesio, Seelos Therapeutics, UCB/Ra Pharma, Woolsey; advisory board fees from Annexon, Bial Biotech, Cortexyme, Chase Therapeutics, Enterin, HillHurst, Merck, nQ Medical, Partner Therapeutics Advisory Board; committee member fees from Biogen, Stoparkinson Healthcare, UCB, Argenx, NeuroSense, Novartis, and Sanofi outside the submitted work. Dr Quintana reported being an employee of Berry Consultants during the conduct of the study. Dr Saville reported being owner of Adaptix Trials outside the submitted work. Dr Detry reported receiving consulting fees from Sean M. Healey & AMG Center for ALS at Mass General and being director and senior statistical scientist at Berry Consultants. Dr Vestrucci reported receiving consulting fees from Sean M. Healey & AMG Center for ALS at Mass General and being a biostatistician at Berry Consultants. Dr Marion reported receiving personal fees from Berry Consultants and being an employee of Berry Consultants during the conduct of the study. Dr McGlothlin reported receiving consulting fees from Sean M. Healey & AMG Center for ALS at Mass General and being a biostatistician at Berry Consultants. Dr Chase reported receiving grants from Biohaven Pharmaceuticals during the conduct of the study. Dr Sherman reported receiving grants from the US Food and Drug Administration (FDA), NIH, Biogen, Mitsubishi Tanabe Pharma America, Amylyx Pharmaceuticals and consulting fees from Mitsubishi Tanabe Pharma America during the conduct of the study. Dr Shefner reported receiving grants from Healey Center for ALS, MPT Pharma America, Amylyx, Sanofi, Novartis, PTC, Neurosense and personal fees from Amylyx, Sanofi, PTC, Acurastem, Neurosense, SwanBIO, and Vertex outside the submitted work. Dr Berry reported receiving grants from Alexion Pharmaceuticals, Rapa Therapeutics, Amylyx Pharmaceuticals, Brainstorm Cell Therapeutics, Biogen, MT Pharma Holdings of America, MTPA; advisor fees from MTPA, Regeneron, MT Pharma Holdings of America, Biogen, Amylyx Pharmaceuticals, Alexion Pharmaceuticals; content contributor fees from Roon; and data safety monitoring board fees from Sanofi outside the submitted work. Dr Giacomelli reported receiving grants from AMG Charitable Foundation, Tackle ALS, The ALS Association, ALS Finding A Cure, Muscular Dystrophy Association, ALS ONE, Arthur M. Blank Family Foundation, I AM ALS, Tambourine ALS Collaborative, and study drug/partial funding from Biohaven during the conduct of the study. Dr Scirocco reported receiving grants from AMG Charitable Foundation, Tackle ALS, The ALS Association, ALS Finding A Cure, Muscular Dystrophy Association, ALS ONE, Arthur M. Blank Family Foundation, I AM ALS, Tambourine ALS Collaborative, and study drug/partial funding from Biohaven during the conduct of the study. Dr Alameda reported being HEALEY Platform trial principal investigator and receiving a stipend during the conduct of the study. Dr Ho reported receiving research funding from Biohaven, Prilenia, RA Pharma, Clene, Seelos, Calico, Denali; funding/advisory board fees from Biogen, Transposon, Sanofi, and Genentech; and research funding from US Army Medical Research Acquisition Activity outside the submitted work. Dr Heitzman reported receiving grants from the HEALEY ALS Platform Trial study, ALS Association, MDA, AbbVie/Calico, Biohaven, Cytokinetics, Denali, Immunovant Sciences, Anelexis, Amylyx, Prilenia, Seelos, NINDS and personal fees from Revance and Amylyx outside the submitted work. Dr Ajroud-Driss reported receiving advisory board and/or speaker fees from Amylyx Pharmaceuticals and Biogen outside the submitted work. Dr Appel reported receiving advisory board, consultant, and/or speaker fees from Coya Therapeutics, Mitsubishi Pharma ALS Biomarkers studies, Eledon, and UCB Pharma and grants from Coya Therapeutics Basic Science studies of Treg single cell transcriptomics outside the submitted work. Dr Shroff reported receiving speaker fees from Almylam, Argenx, AstraZeneca, and UCB outside the submitted work. Dr Maragakis reported receiving study funding from Massachusetts General Hospital, Biogen, Cytokinetics; personal fees from Nura Bio and Novartis; and nonfinancial support from Janssen and Akava outside the submitted work. Dr Simmons reported receiving monitoring committee and/or consultation fees from Biogen, Corcept, Insmed, and Columbia University and grants from Clene during the conduct of the study. Dr Goutman reported receiving grants from Healey Center at MGH during the conduct of the study. Dr Miller reported receiving study resources from Healey Center at MGH Resources to complete the study through the HEALEY Platform Trial during the conduct of the study; consulting, honoraria, and/or licensing fees from Ionis Pharmaceuticals, Biogen, Arbor Bio, C2N, and Denali outside the submitted work. Dr Fernandes reported receiving grants from MGH philanthropy (Clene, Seelos, UCB, Biohaven, Prilenia, Denali Therapeutics, Calico Life Sciences) during the conduct of the study; personal fees from Columbia University (Tsumura & Co) Medical Monitor, grants from Columbia University, and grants from PTC Therapeutics (site principal investigator) outside the submitted work. Dr Ilieva reported receiving grants from ALSA, Akcea, and research support from Vickie and Jack Farber outside the submitted work. Dr Weiss reported receiving advisory board member and/or data safety monitoring board fees from Biogen, Cytokinetics, Misubishi Tanabe, Amylyx, and Sanofi during the conduct of the study. Dr Foster reported receiving grants from Sean M. Healey and AMG Center for ALS during the conduct of the study. Dr Vu reported receiving research grants from Alector, Alexion, AstraZeneca Rare Disease, Argenx, Amylyx Pharma, Annexon, Apellis, CSL Behring, Biogen, Cytokinetics, Dianthus, Harmony/Viela Bio, HEALEY Platform Trials, Mitsubishi Tanaka, RA/UCB, Sanofi, Momenta/Janssen, and Woolsey Pharma during the conduct of the study and personal fees for serving on speaking bureaus and for consulting work unrelated to ALS and the Healey trials. Dr Owegi reported receiving funding from HEALEY Platform during the conduct of the study. Dr Arcila-Londono reported receiving a clinical trials grant from the ALS Association. Dr Jackson reported receiving grants from MGH during the conduct of the study. Dr Heiman-Patterson reported receiving grants from the HEALEY Platform Trial, MT Pharma, Amylyx, and the ALS Association and advisory board fees from MT Pharma, Amylyx, and Novartis outside the submitted work. Dr Lewis reported receiving consultant/advisory board fees from Alexion, Annexon, Argenx, Avilar, BioCryst, CSL Behring, Dianthus, Grifols, J&J, Immunovant, Nervosave, Nuvig, Sanofi, Seismic, and Takeda; data safety monitoring board fees from Boehringer Ingleheim and Intellia; medical advisory board fees from GBS-CIDP Foundation International; speaker fees from Medscape; and royalties from UpToDate. Dr Walk reported receiving personal fees from Mitsubishi Tanabe Pharma America, Amylyx Pharmaceuticals, Biogen, and Massachusetts General Hospital outside the submitted work. Dr Johnson reported receiving grants from Massachusetts General Hospital during the conduct of the study and speaker fees from Argenx outside the submitted work. Dr Elliott reported receiving grants from Biohaven, the ALS Association, Denali Therapeutics, UCB, Clene, Prilenia Therapeutics, Medicinova, and AbbVie outside the submitted work. Dr Rutkove reported receiving equity ownership from Myolex, Haystack Diagnostics and consultant fees from Eikonoklastes Therapeutics, Cytokinetics, and Takeda Pharmaceuticals outside the submitted work. Dr McIlduff reported having a Massachusetts General Hospital master site clinical trial agreement during the conduct of the study. Dr Bedlack reported receiving grants from MGH Healey Center, CReaTe Consortium, and Medicinova and personal fees from AB Science, ALS Association, American Institute of Biological Sciences, Amylyx, Betty’s Brigade, Biogen Idec, Carespace Health, Clene Nanomedicine, Eikonoklastes, Eisenhower Medical Center, General Dynamics Information Technology, GenieUS, Guidepoint Global, Ionis Pharmaceuticals, Kaplan, MJH Life Sciences, Neurosense, Novartis, PTC Therapeutics, Prime Education, Roon, Springer Publishing, State of Maryland, The CM Group, Uniqure, and Webb MD outside the submitted work. Dr Goyal reported receiving grants from the HEALEY Platform, Abcuro, Amylyx, Annexon, Calico, Clene, Cytokinetics, Fulcrum, Janssen, Kezar, Medicinova, MT Pharma, PepGen, PTC, Roche, Sanofi, and Transposon outside the submitted work. Dr Benatar reported receiving per-patient reimbursement from MGH; consulting fees from Prilenia, Alector, Biogen, Novartis, BMS, Woolsey, Eli Lilly, uniQure, and Arrowhead; and having a patent for UMIP-142A pending. Dr Beydoun reported receiving grants from Sean Healey AMG Center for ALS, Mass General, AB Science, Abcuro, Genentech, Immunovant, Regeneron, RemeGen, Sanofi; personal fees from Alexion, Alnylam, Amgen, Argenx, AstraZeneca, Janssen, CSL, Takeda, and Grifols outside the submitted work. Dr Shah reported grants from Muscular Dystrophy Association, Argenx, and Abcuro outside the submitted work. Dr Granit reported being an employee of Biohaven Pharmaceuticals during the conduct of the study and advisory board fees from Amylyx Pharmaceuticals outside the submitted work. Dr Grossman reported being an employee of and shareholder in Biohaven during the conduct of the study. Drs Campbell and Qureshi reported receiving a salary and stock options from Biohaven during the conduct of the study. Dr Cudkowicz reported receiving grants from Biohaven; consulting fees from Transposon Therapeutics, Ono Pharmaceuticals, BioScience, Quralis, Novartis, Neurosense, Biogen, Locustwalk, Pasitnea, Roche, Denali, Immunity Pharma, RRD, Takeda, MTPC, Arrowhead, Vector Servier, Eledon, Cytokinetics, Regeneron, Infelctis BioScience; and board member fees from Praxis outside the submitted work. Dr Babu reported receiving grants from Biogen, Ionis, OrphAI, Denali, Novartis and consulting fees from Uniqure and MarvelBiome outside the submitted work. No other disclosures were reported.
Figures
Comment in
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

