A Systematic Review and Meta-analysis of the Effectiveness of Remdesivir to Treat SARS-CoV-2 in Hospitalized Patients: Have the Guidelines Evolved With the Evidence?
- PMID: 40067859
- PMCID: PMC12314504
- DOI: 10.1093/cid/ciaf111
A Systematic Review and Meta-analysis of the Effectiveness of Remdesivir to Treat SARS-CoV-2 in Hospitalized Patients: Have the Guidelines Evolved With the Evidence?
Abstract
Background: With progressive accumulation of knowledge on SARS-CoV-2 infection clinical management, treatment guidelines recommended several options including remdesivir, a broad-spectrum antiviral. Given the evolving nature of coronavirus disease 2019, capturing the totality of scientific evidence from clinical trials and observational studies is critical to inform clinical decision making. We conducted a systematic literature review with meta-analysis to summarize the effectiveness of remdesivir among hospitalized adults.
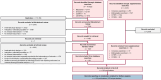
Methods: We systematically searched MEDLINE, Embase and Cochrane Library databases for interventional and observational studies examining remdesivir efficacy. A rigorous double-reviewer approach was used for source identification, screening, data extraction and risk of bias assessment. A hierarchical random-effects model meta-analysis was used, with subgroup analyses for randomized controlled trials (RCTs) and real-world (RW) studies.
Results: From January 2019 to December 2023 >18 000 sources were screened, and 122 unique studies were identified, reporting on 25 174 participants in RCTs and 1 279 859 in RW studies. Remdesivir significantly increased survival in the overall population (odds ratio, 0.69 [95% confidence interval, .55-.86]; P = .001] across SARS-CoV-2 variants and disease severity levels: no supplemental oxygen (0.81 [.75-.88]), low-flow oxygen (0.71 [.64-.79]), high-flow oxygen (0.87 [.83-.91]), and invasive mechanical ventilation (0.78 [.68-.90]). Rehospitalization risk was significantly reduced in patients receiving remdesivir (odds ratio, 0.72 [95% confidence interval, .64-.81]).
Conclusions: Our comprehensive systematic literature review, capturing the totality of evidence, showed a significant survival benefit among patients hospitalized for SARS-CoV-2 infection and receiving remdesivir, across all disease severity levels. To assure that healthcare providers are aware of and deploy evidence-based optimal care, recommendations should rely on both RCT and RW data.
Keywords: COVID-19; SARS-CoV-2; effectiveness; remdesivir; treatment guidelines.
© The Author(s) 2025. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. M. B. has been a consultant to AdvanzPharma, AstraZeneca, Gilead, MSD, and Pfizer and has received honoraria from AdvanzPharma, AstraZeneca, Biomerieux, Gilead, MSD, and Pfizer. E. M., T. F. O., and M. C. are employees and shareholders of Gilead Sciences. A. N. A. has been a consultant to Alexion, Aseptiscope, AstraZeneca, Bayer, Dexcom, Eli Lilly, Ferring, Gilead, GSK, HeartRite, Novo Nordisk, Pfizer, Renibus, Reprieve, Salix, Seres, and Spero and has received grants from the National Institutes of Health/National Institute of Allergy and Infectious Diseases, NeuroRx Pharma, Pulmotect, Blade Therapeutics, Novartis, Takeda, Humanigen, Eli Lilly, PTC Therapeutics, OctaPharma, Fulcrum Therapeutics, and Alexion, unrelated to this work. Y. D. has been a consultant to AbbVie, GSK, MeijiSeika Pharma, Moderna, Pfizer, and Shionogi & Co and has received grants from Entasis and honoraria from Shionogi, BD, and Gilead. P. L. received honoraria from AstraZeneca, Moderna, and Pfizer. C. G. R. is a board member of Gilead Sciences. M. R. has received honoraria from Gilead Sciences. A. R. and E. K. are employees of Costello Medical, which received payment from Gilead Sciences for analytical services for the current study. M. V. N. and S. F. are employees of Certara USA, a consulting firm that received funding from Gilead Sciences. P. E. S. received grants from Gilead and ViiV and consulting fees from Gilead, Merck and ViiV. A. C. K. received grants from the National Institutes of Health. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



References
-
- Benavidez G, Frakt AB. 2019. Fixing clinical practice guidelines. Health Affairs Forefront. Available at: https://www.healthaffairs.org/do/10.1377/forefront.20190730.874541/full/. Accessed 16 December 2024. - DOI
-
- Kalil AC. Treating COVID-19-off-label drug use, compassionate use, and randomized clinical trials during pandemics. JAMA 2020; 323:1897–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

