Biotransforming the "Forever Chemicals": Trends and Insights from Microbiological Studies on PFAS
- PMID: 40067878
- PMCID: PMC11948467
- DOI: 10.1021/acs.est.4c04557
Biotransforming the "Forever Chemicals": Trends and Insights from Microbiological Studies on PFAS
Abstract
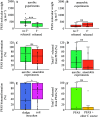
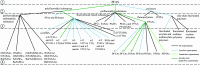
Per- and polyfluoroalkyl substances (PFAS) are recalcitrant contaminants of emerging concern. Research efforts have been dedicated to PFAS microbial biotransformation in the hopes of developing treatment technologies using microorganisms as catalysts. Here, we performed a meta-analysis by extracting and standardizing quantitative data from 97 microbial PFAS biotransformation studies and comparing outcomes via statistical tests. This meta-analysis indicated that the likelihood of PFAS biotransformation was higher under aerobic conditions, in experiments with defined or axenic cultures, when high concentrations of PFAS were used, and when PFAS contained fewer fluorine atoms in the molecule. This meta-analysis also documented that PFAS biotransformation depends on chain length, chain branching geometries, and headgroup chemistry. We found that the literature is scarce or lacking in (i) anaerobic PFAS biotransformation experiments with well-defined electron acceptors, electron donors, carbon sources, and oxidation-reduction potentials, (ii) analyses of PFAS biotransformation products, and (iii) analyses to identify microorganisms and enzymes responsible for PFAS biotransformation. To date, most biotransformation research emphasis has been on 8:2 fluorotelomer alcohol (8:2 FTOH), 6:2 fluorotelomer alcohol (6:2 FTOH), perfluorooctanesulfonic acid (PFOS), and perfluorooctanoic acid (PFOA). A wide array of PFAS remains to be tested for their potential to biotransform.
Keywords: PFAS; bacteria; biodegradation; bioremediation; biotransformation; defluorination; meta-analysis; microbial; perfluoroalkyl; polyfluoroalkyl.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Olsen G. W.; Mair D. C.; Lange C. C.; Harrington L. M.; Church T. R.; Goldberg C. L.; Herron R. M.; Hanna H.; Nobiletti J. B.; Rios J. A.; et al. Per- and Polyfluoroalkyl Substances (PFAS) in American Red Cross Adult Blood Donors, 2000–2015. Environ. Res. 2017, 157, 87–95. 10.1016/j.envres.2017.05.013. - DOI - PubMed
-
- Scher D. P.; Kelly J. E.; Huset C. A.; Barry K. M.; Hoffbeck R. W.; Yingling V. L.; Messing R. B. Occurrence of Perfluoroalkyl Substances (PFAS) in Garden Produce at Homes with a History of PFAS-contaminated Drinking Water. Chemosphere 2018, 196, 548–555. 10.1016/j.chemosphere.2017.12.179. - DOI - PubMed
-
- Liu X.; Fang M.; Xu F.; Chen D. Characterization of the Binding of Per- and Poly-Fluorinated Substances to Proteins: A Methodological Review. Trends Environ. Anal. Chem. 2019, 116, 177–185. 10.1016/j.trac.2019.05.017. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

