Epitope-directed selection of GPCR nanobody ligands with evolvable function
- PMID: 40067891
- PMCID: PMC11929449
- DOI: 10.1073/pnas.2423931122
Epitope-directed selection of GPCR nanobody ligands with evolvable function
Abstract
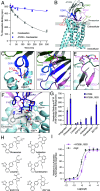
Antibodies have the potential to target G protein-coupled receptors (GPCRs) with high receptor, cellular, and tissue selectivity; however, few antibody ligands for GPCRs exist. Here, we describe a generalizable selection method to enrich for GPCR ligands from a synthetic camelid antibody fragment (nanobody) library. Our strategy yielded multiple nanobody ligands for the angiotensin II type I receptor (AT1R), a prototypical GPCR and important drug target. We found that nanobodies readily act as allosteric modulators, encoding selectivity for both the receptor and chemical features of GPCR ligands. We then used structure-guided design to convert two nanobodies from allosteric ligands to competitive AT1R inhibitors through simple mutations. This work demonstrates that nanobodies can encode multiple pharmacological behaviors and have great potential as evolvable scaffolds for the development of next-generation GPCR therapeutics.
Keywords: GPCR; allostery; angiotensin; cryo-EM; nanobody.
Conflict of interest statement
Competing interests statement:A.C.K. is a cofounder and consultant for biotechnology companies Tectonic Therapeutic and Seismic Therapeutic, and also for the Institute for Protein Innovation, a nonprofit research institute. A.C.K. holds stock in Tectonic Therapeutic and Seismic Therapeutic.
Figures



References
-
- Hutchings C. J., Koglin M., Olson W. C., Marshall F. H., Opportunities for therapeutic antibodies directed at G-protein-coupled receptors. Nat. Rev. Drug Discov. 16, 787–810 (2017). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

