NEMO is essential for directing the kinases IKKα and ATM to the sites of DNA damage
- PMID: 40067909
- PMCID: PMC12070652
- DOI: 10.1126/scisignal.adr0128
NEMO is essential for directing the kinases IKKα and ATM to the sites of DNA damage
Abstract
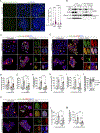
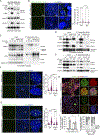
The DNA damage repair kinase ATM is phosphorylated by the NF-κB pathway kinase IKKα, resulting in enhanced DNA damage repair through the nonhomologous end-joining pathway. Thus, inhibition of IKKα enhances the efficacy of cancer therapy based on inducing DNA damage. Here, we found a role for the IKK regulatory subunit NEMO in DNA damage repair mediated by ATM and IKKα. Exposure to damaging agents induced the interaction of NEMO with a preformed ATM-IKKα complex, which was required to target active ATM and IKKα to chromatin for efficient DNA damage repair but not for activating ATM. Recognition of damaged DNA by the IKKα-NEMO-ATM complex was facilitated by the interaction between NEMO and histones and depended on the ADP ribosylation of histones by the enzyme PARP1. NEMO-deficient cells showed increased activity of the kinase ATR, and inhibition of ATR potentiated the effect of chemotherapy in cells lacking NEMO or IKKα. Bioinformatic analysis of colorectal cancer datasets demonstrated that the expression of genes encoding IKKα, NEMO, and ATM correlated with poor patient prognosis, suggesting that the mechanism linking these three elements may be clinically relevant.
Conflict of interest statement
Figures




References
-
- Touil Y, Igoudjil W, Corvaisier M, Dessein A-F, Vandomme J, Monté D, Stechly L, Skrypek N, Langlois C, Grard G, Millet G, Leteurtre E, Dumont P, Truant S, Pruvot F-R, Hebbar M, Fan F, Ellis LM, Formstecher P, Van Seuningen I, Gespach C, Polakowska R, Huet G, Colon Cancer Cells Escape 5FU Chemotherapy-Induced Cell Death by Entering Stemness and Quiescence Associated with the c-Yes/YAP Axis. Clin. Cancer Res. 20, 837–846 (2014). - PMC - PubMed
-
- Dhimolea E, de Matos Simoes R, Kansara D, Al’Khafaji A, Bouyssou J, Weng X, Sharma S, Raja J, Awate P, Shirasaki R, Tang H, Glassner BJ, Liu Z, Gao D, Bryan J, Bender S, Roth J, Scheffer M, Jeselsohn R, Gray NS, Georgakoudi I, Vazquez F, Tsherniak A, Chen Y, Welm A, Duy C, Melnick A, Bartholdy B, Brown M, Culhane AC, Mitsiades CS, An Embryonic Diapause-like Adaptation with Suppressed Myc Activity Enables Tumor Treatment Persistence. Cancer Cell 39, 240–256.e11 (2021). - PMC - PubMed
-
- Rehman SK, Haynes J, Collignon E, Brown KR, Wang Y, Nixon AML, Bruce JP, Wintersinger JA, Singh Mer A, Lo EBL, Leung C, Lima-Fernandes E, Pedley NM, Soares F, McGibbon S, He HH, Pollet A, Pugh TJ, Haibe-Kains B, Morris Q, Ramalho-Santos M, Goyal S, Moffat J, O’Brien CA, Colorectal Cancer Cells Enter a Diapause-like DTP State to Survive Chemotherapy. Cell, doi: 10.1016/j.cell.2020.11.018 (2021). - DOI - PMC - PubMed
-
- Solé L, Lobo-Jarne T, Villanueva A, Vert A, Guillén Y, Sangrador I, Barbachano A, Lop J, Guix M, Salido M, Bellosillo B, García-Romero R, Garrido M, González J, Martínez-Iniesta M, Lopez-Arribillaga E, Salazar R, Montagut C, Torres F, Iglesias M, Celià-Terrassa T, Muñoz A, Bigas A, Espinosa L, Chemotherapy induces a YAP1-dependent fetal conversion to human Colorectal Cancer cells that is predictive of poor patient outcome. bioRxiv (2021).
-
- Cánovas B, Igea A, Sartori AA, Gomis RR, Paull TT, Isoda M, Pérez-Montoyo H, Serra V, González-Suárez E, Stracker TH, Nebreda AR, Targeting p38α Increases DNA Damage, Chromosome Instability, and the Anti-tumoral Response to Taxanes in Breast Cancer Cells. Cancer Cell, doi: 10.1016/j.ccell.2018.04.010 (2018). - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

