M6A Modified miR-31-5p Suppresses M1 Macrophage Polarization and Autoimmune Dry Eye by Targeting P2RX7
- PMID: 40068094
- PMCID: PMC12061282
- DOI: 10.1002/advs.202415341
M6A Modified miR-31-5p Suppresses M1 Macrophage Polarization and Autoimmune Dry Eye by Targeting P2RX7
Abstract
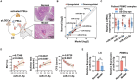
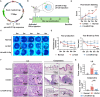
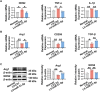
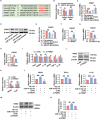
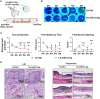
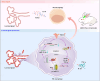
The dysregulation of the M1/M2 macrophage balance plays a pivotal role in autoimmune diseases. However, the interplay between microRNAs (miRNAs) and N6-methyladenosine (m6A) modulation in regulating this balance remains poorly understood. Here, a significant reduction in miR-31-5p levels is observed in the lacrimal glands of rabbit autoimmune dacryoadenitis and the peripheral blood mononuclear cells (PBMCs) of Sjögren's syndrome (SS) dry eye patients. Overexpression of miR-31-5p exhibits preventive and therapeutic effects on rabbit autoimmune dacryoadenitis. Further investigation revealed that miR-31-5p overexpression significantly restored the M1/M2 macrophage balance both in vivo and in vitro. Mechanistically, miR-31-5p directly targets the P2x7 receptor (P2RX7), leading to the inactivation of p38 mitogen-activated protein kinases (MAPK) signaling and reduced expression of M1 markers. Furthermore, methylated RNA immunoprecipitation and luciferase reporter assays demonstrated that fat mass and obesity-associated protein (FTO)-mediated m6A demethylation, which sustains pri-miR-31 stability, is responsible for the decreased miR-31-5p levels in autoimmune dry eye. Notably, PBMC samples from SS dry eye patients further support the link between reduced miR-31-5p levels and M1 macrophage activation observed in rabbits. Overall, these data highlight the critical role of the FTO/miR-31-5p/P2RX7/p38 MAPK axis in autoimmune inflammation, suggesting their potential as therapeutic targets for autoimmune dry eye.
Keywords: m6A; macrophage polarization; miR‐31‐5p; p2x7 receptor; sjogren's syndrome dry eye.
© 2025 The Author(s). Advanced Science published by Wiley‐VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
MeSH terms
Substances
Grants and funding
- 82070929/National Natural Science Foundation of China
- 81970793/National Natural Science Foundation of China
- TJYXZDXK-037A/Tianjin Key Medical Discipline (Specialty) Construction Project
- 2023ZD018/The Science & Technology Development Fund of Tianjin Education Commission for Higher Education
- YDYYRCXM-B2023-3/Tianjin Medical University Eye Hospital High-Level innovative Talents Programme
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
