Macrophage-Specific Progranulin Deficiency Prevents Diet-Induced Obesity through the Inhibition of Hypothalamic and Adipose Tissue Inflammation
- PMID: 40068621
- PMCID: PMC12270572
- DOI: 10.4093/dmj.2024.0486
Macrophage-Specific Progranulin Deficiency Prevents Diet-Induced Obesity through the Inhibition of Hypothalamic and Adipose Tissue Inflammation
Abstract
Backgruound: Chronic low-grade inflammation in multiple metabolic organs contributes to the development of insulin resistance induced by obesity. Progranulin (PGRN) is an evolutionarily-conserved secretory protein implicated in immune modulation. The generalized deletion of the PGRN-encoded Grn gene improves insulin resistance and glucose intolerance in obese mice fed a high-fat diet (HFD). However, it remains unclear which cells or organs are responsible for the beneficial metabolic effect of Grn depletion.
Methods: Considering the critical role of macrophages in HFD-induced obesity and inflammation, we generated mice with a macrophage-specific Grn depletion (Grn-MΦKO mice) by mating lysozyme M (LysM)-Cre and Grn-floxed mice. Body weight, food intake, energy expenditure, and glucose and insulin tolerance were compared between Grn-MΦKO mice and their wildtype (WT) controls under normal chow diet (NCD)- or HFD-fed conditions. We also examined macrophage activation and inflammation- related gene expression in the visceral adipose tissue and hypothalamus along with insulin and leptin signaling.
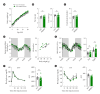
Results: Grn-MΦKO mice showed no alteration in metabolic phenotypes under NCD-fed conditions. However, upon HFD feeding, these mice exhibited less weight gain and improved glucose and insulin tolerance compared to WT mice. Moreover, HFD-induced macrophage activation and proinflammatory cytokine expression were significantly reduced in both the adipose tissue and hypothalamus of Grn-MΦKO mice, while HFD-induced impairments in leptin and insulin signaling showed improvement.
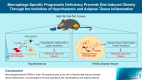
Conclusion: Macrophage-derived PGRN and possibly other Grn products play a critical role in the development of HFD-induced obesity, tissue inflammation, and impaired hormonal signaling in both central and peripheral metabolic organs.
Keywords: Adipose tissue; Hypothalamus; Inflammation; Macrophages; Metabolic diseases; Obesity; Progranulins.
Conflict of interest statement
No potential conflict of interest relevant to this article was reported.
Figures



References
-
- Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. 2017;13:633–43. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

