Complex water networks visualized by cryogenic electron microscopy of RNA
- PMID: 40068818
- PMCID: PMC12137144
- DOI: 10.1038/s41586-025-08855-w
Complex water networks visualized by cryogenic electron microscopy of RNA
Abstract
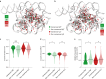
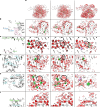
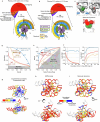
The stability and function of biomolecules are directly influenced by their myriad interactions with water1-16. Here we investigated water through cryogenic electron microscopy (cryo-EM) on a highly solvated molecule: the Tetrahymena ribozyme. By using segmentation-guided water and ion modelling (SWIM)17,18, an approach combining resolvability and chemical parameters, we automatically modelled and cross-validated water molecules and Mg2+ ions in the ribozyme core, revealing the extensive involvement of water in mediating RNA non-canonical interactions. Unexpectedly, in regions where SWIM does not model ordered water, we observed highly similar densities in both cryo-EM maps. In many of these regions, the cryo-EM densities superimpose with complex water networks predicted by molecular dynamics, supporting their assignment as water and suggesting a biophysical explanation for their elusiveness to conventional atomic coordinate modelling. Our study demonstrates an approach to unveil both rigid and flexible waters that surround biomolecules through cryo-EM map densities, statistical and chemical metrics, and molecular dynamics simulations.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures











Update of
-
Complex Water Networks Visualized through 2.2-2.3 Å Cryogenic Electron Microscopy of RNA.bioRxiv [Preprint]. 2025 Jan 24:2025.01.23.634578. doi: 10.1101/2025.01.23.634578. bioRxiv. 2025. Update in: Nature. 2025 Jun;642(8066):250-259. doi: 10.1038/s41586-025-08855-w. PMID: 39896454 Free PMC article. Updated. Preprint.
References
-
- Hermann, T. & Patel, D. J. Stitching together RNA tertiary architectures. J. Mol. Biol.294, 829–849 (1999). - PubMed
-
- Auffinger, P. & Westhof, E. Hydration of RNA base pairs. J. Biomol. Struct. Dyn.16, 693–707 (1998). - PubMed
-
- Egli, M., Portmann, S. & Usman, N. RNA hydration: a detailed look. Biochemistry35, 8489–8494 (1996). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

