British Society of Gastroenterology practice guidance on the management of acute and chronic gastrointestinal symptoms and complications as a result of treatment for cancer
- PMID: 40068855
- PMCID: PMC12322484
- DOI: 10.1136/gutjnl-2024-333812
British Society of Gastroenterology practice guidance on the management of acute and chronic gastrointestinal symptoms and complications as a result of treatment for cancer
Abstract
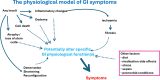
Background: Survival rates after a diagnosis of cancer are improving. Poorly managed gastrointestinal (GI) side effects can interfere with delivery of curative cancer treatment. Long-term physical side effects of cancer therapy impinge on quality of life in up to 25% of those treated for cancer, and GI side effects are the most common and troublesome.
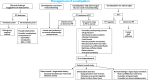
Aim: To provide comprehensive, practical guidance on the management of acute and chronic luminal gastrointestinal symptoms arising during and after treatment for cancer METHODS: A multidisciplinary expert group including patients treated for cancer, divided into working parties to identify, and synthesise recommendations for the optimal assessment, diagnosis and appropriate interventions for luminal GI side effects of systemic and local cancer therapies. Recommendations were developed using the principles of the BMJ AGREE II reporting.
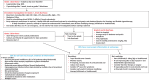
Results: 103 recommendations were agreed. The importance of the patient perspective and what can be done to support patients are emphasised. Key physiological principles underlying the development of GI toxicity arising from cancer therapy are outlined. Individual symptoms or symptom clusters are poor at distinguishing the underlying cause(s), and investigations are required if empirical therapy does not lead rapidly to significant benefits. Patients frequently have multiple GI causes for symptoms; all need to be diagnosed and optimally treated to achieve resolution. Investigations and management approaches now known to be ineffective or of questionable benefit are highlighted.
Conclusions: The physical, emotional and financial costs to individuals, their families and society from cancer therapy can be considerable. Identifying and signposting affected patients who require specialist services is the role of all clinicians. Progress in the treatment of cancer increasingly means that patients require expert, multidisciplinary supportive care providing effective and safe treatment at every stage of the cancer journey. Development of such expertise should be prioritised as should the education of health professionals and the public in what, when and how acute and chronic gastrointestinal symptoms and complications should be managed.
Keywords: bleeding; cancer; chemotherapy; diarrhoea; radiotherapy.
© Author(s) (or their employer(s)) 2025. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ Group.
Conflict of interest statement
Competing interests: None declared.
Figures





References
-
- Khalid U, McGough C, Hackett C, et al. A modified inflammatory bowel disease questionnaire and the Vaizey Incontinence questionnaire are more sensitive measures of acute gastrointestinal toxicity during pelvic radiotherapy than RTOG grading. Int J Radiat Oncol Biol Phys. 2006;64:1432–41. doi: 10.1016/j.ijrobp.2005.10.007. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
